Abstract
Currently, a number of novel hemophilia drugs with different modes of action are being developed for either subcutaneous (sc) and/or less frequent administration as compared to current intravenous (iv) factor replacement therapy. These drugs may provide a major improvement of hemophilia treatment.
Tissue factor pathway inhibitor (TFPI) down-regulates the initiation of coagulation by inhibition of the factor VIIa/tissue factor/factor Xa (FVIIa/TF/FXa) complex. Abrogation of TFPI function extends FX activation within the initiation phase of the clotting system and may potentially completely bypass the need for factor VIII (FVIII) and factor IX (FIX). Concizumab (previously mAb 2021) is a high-affinity humanized monoclonal antibody against the Kunitz 2 domain of TFPI, intended for sc prophylaxis in hemophilia A and B patients with and without inhibitors, and currently being tested in clinical phase 1/2 trials. Concizumab has been shown to effectively prevent inhibition of FVIIa/TF/FXa and decrease cuticle bleeding in a dose-dependent manner in hemophilic rabbits. Blood loss in hemophilic rabbits was reduced for 7 days after administration of a single high dose of concizumab. It is expected that the inhibition of TFPI is effective as long as the initiation complex FVIIa/TF/FXa is active. In the present study, it was evaluated if concizumab can reduce an ongoing bleeding following administration at different time points after induction of a bleeding in a rabbit hemophilia model.
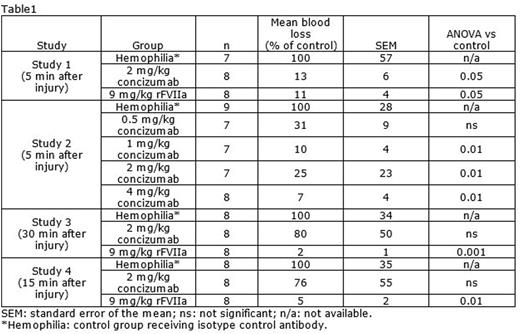
Four separate cuticle bleeding studies in rabbits made hemophilic by iv administration of a monoclonal human FVIII-antibody were performed. In the first study, administration of 2 mg/kg concizumab iv reduced cuticle blood loss to a similar extent as 9 mg/kg recombinant activated factor VII (rFVIIa) iv when dosed 5 minutes after induction of bleeding, with a blood loss of 13 and 11%, respectively, compared to a control group receiving isotype control antibody (n=7-8; p<0.05 for both; Table 1). In a subsequent dose-response study, 0.5, 1, 2 and 4 mg/kg concizumab (iv) dosed 5 minutes after induction of bleeding reduced blood loss to 31, 10, 25 and 7% of control level, respectively (n=7-9; p<0.01 for 1, 2 and 4 mg/kg; Table 1).
The ability of concizumab to reduce blood loss when dosed 30 minutes after induction of bleeding was further evaluated. Rabbits received 2 mg/kg concizumab iv and blood loss was 80% of the blood loss in hemophilic control animals (n=8; not significant), whereas 9 mg/kg rFVIIa iv reduced blood loss to 2% of control level (p<0.001; Table 1).
Finally, 2 mg/kg concizumab, administered iv to rabbits 15 minutes after induction of bleeding, resulted in a blood loss of 76% compared to hemophilic control animals (n=8; not significant), whereas 2 mg/kg rFVIIa iv reduced blood loss to 5% of control level (p<0.01; Table 1).
Neutralization of TFPI will sustain the activity of the TF/FVIIa/FXa initiation complex. As this complex is present only for a short period of time when the blood vessel wall is injured and TF is exposed, concizumab is not expected to be useful for treatment of an ongoing bleeding. Here, we show that concizumab is as potent as rFVIIa in an ongoing bleeding in hemophilic rabbits, when administrated 5 minutes after bleeding induction. In contrast, no effect of concizumab was observed when administered 15 or 30 minutes after induction of bleeding, whereas rFVIIa significantly reduced blood loss when dosed at these time-points. These data support a TF-dependent effect of concizumab and a TF-independent effect of pharmacological doses of rFVIIa.
Lauritzen:Novo Nordisk: Employment. Hilden:Novo Nordisk: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal