Abstract
Introduction: Human ADAMTS13 has metalloprotease (M), disintegrin-like (D), thrombospondin-1 (T), Cys-rich (C) and spacer (S) domains (proximal domains), followed by 7 T and 2 CUB domains (distal domains).ADAMTS13 regulates von Willebrand factor (VWF) by cleaving a Tyr-Met bond in the VWF A2 domain that is exposed under shear force. Acquired or inherited ADAMTS13 deficiency impairs this regulatory mechanism and cause thrombotic thrombocytopenic purpura (TTP), a life threatening disorder. We recently showed that ADAMTS13 is autoinhibited by its distal T8-CUB domains and this inhibition is relieved by binding to VWF. Thus, VWF promotes its own destruction by allosterically activating ADAMTS13. We investigated the evolution and conservation of this remarkable regulatory mechanism.
Methods:ADAMTS13 cDNA sequences for >300 species were assembled from genome assemblies and SRA transcriptomic and genomic data. Recombinant ADAMTS13 variants were expressed in HEK293 cells and purified chromatographically. Plasma was obtained from dog, armadillo, rabbit, primates (human, baboon, marmoset, macaque), rodents (rat, mouse), ungulates (cattle, sheep, pig, horse), birds (chicken, pigeon), and Xenopus laevis. Plasma ADAMTS13 activity of at least three members of each species was assayed with human-FRETS71, a fluorogenic ADAMTS13 substrate based on human VWF. Similar fluorogenic substrates were prepared with armadillo, rat, pigeon and cattle VWF sequences. Plasma ADAMTS13 also was assayed with allosteric activators of human ADAMTS13: monoclonal antibodies (MAbs) against distal T and CUB domains, low pH (pH 6), and recombinant human VWF D4 domain. ADAMTS13-VWF interactions were assessed with biolayer interferometry (BLI) and binding data fitted to 1:1 binding model.
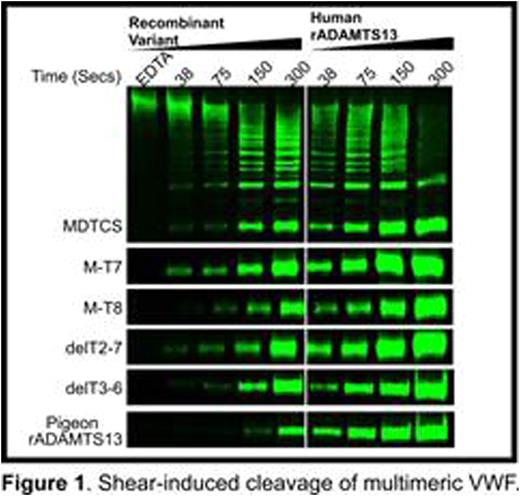
Results: The ancestral ADAMTS13 had a long propeptide that is characteristic of most metalloproteases but notably missing from human ADAMTS13, and 8 distal T domains compared to 7 distal T domains in human ADAMTS13. The ancestral structure today is found in most reptiles, amphibians, and marsupials. The propeptide, T3, T4, T6 and T6a have been deleted independently several times in mammals, birds, amphibians and fish. Most animal plasmas cleaved human-FRETS71 less rapidly than human plasma, although armadillo, rabbit, and dog were 2.4-, 3.0-, 4.0-fold more active, respectively. Human and cattle VWF have Tyr-Met cleavage sites, but armadillo VWF has a Tyr-Leu bond. Armadillo plasma cleaved armadillo-FRETS71 and human-FRETS71 at a similar rate; other species cleaved armadillo-FRETS71 15% to 88% as rapidly as human-FRETS71. Surprisingly, cattle-FRETS71 was cleaved ~2- to 15-fold faster than human-FRETS71 by most plasmas. Except for C57Bl/6 mice (which have ADAMTS13 truncated after T6), armadillo, and dog, all plasmas screened were activated by MAbs or VWF D4. Frog and pigeon ADAMTS13 were activated ~6-fold by D4, whereas human ADAMTS13 was activated only 2-fold. Absence of CUB domains in armadillo ADAMTS13 abolished activation by D4. In contrast, absence of all but 3 distal T domains in pigeon ADAMTS13 did not impair activation by VWF D4. Recombinant human ADAMTS13 constructed with deletions like those in armadillo and pigeon ADAMTS13 had similar properties: delT3-6 was activated by VWF D4 but M-T8 was not. All enzymes activated by VWF D4 showed enhanced cleavage of multimeric VWF under fluid shear stress compared to enzymes that are not activated by VWF D4 (Figure 1 and Table 1). When normalized for activity toward FRETS71, pigeon ADAMTS13 was ~2-fold more specific for VWF multimers than is human ADAMTS13. In agreement with the cleavage data, recombinant human and pigeon ADAMTS13, and MT-8 bound VWF D4-CK with Kd values in the 350-840 nM range, whereas MDTCS, MT-7 and delT2-7 did not bind.
Conclusion: Allosteric regulation of ADAMTS13 by VWF is broadly conserved, with some apparent exceptions. The distal T3-T6 domains are dispensable for allosteric regulation, whereas T7, T8 and CUB domains are required. Furthermore, ADAMTS13 from different species exhibits striking variation in substrate specificity. For most species, altering the sequence of the VWF substrate can markedly increase the rate of cleavage, indicating that ADAMTS13 and VWF have not evolved to be optimal enzyme-substrate pairs. These properties are likely to reflect evolutionary pressure to balance the risk of VWF-dependent bleeding and thrombosis.
Sadler:Ablynx: Consultancy; 23andMe: Consultancy; BioMarin: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal