Abstract
Autoimmune lymphoproliferative syndrome (ALPS) is a rare disorder of the immune system due to defective Fas-mediated apoptosis which usually presents in childhood with lymphadenopathy, hypersplenism, hypergammaglobulinemia and multilineage cytopenias. Heterozygous germline mutations in FAS (TNFRSF6) gene commonly cause what is known as ALPS-FAS. Patients with genetic defect in Caspase 10 are classified as ALPS-CASP10. We have identified 16 individuals from 11 families with caspase 10 mutations. However six individuals were noted to have recognized caspase 10 polymorphisms. Here we are describing the clinical spectrum of a cohort of 10 patients from 8 families seen in our institution during the last 20 years with features suggestive of ALPS due to Caspase 10 mutations.
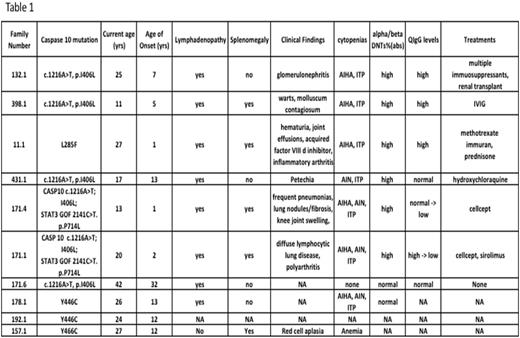
There were 5 males and 5 females. Their median age at presentation was 7 years (Range 1-32yrs). Lymphadenopathy (n=8) was noted in most and splenomegaly in 5/10. The immunophenotypic signature of ALPS, "double negative" TCR-__+ T lymphocytes (DNTs) that lack both CD4 and CD8 markers were expanded in 6/8 patients. One patient (#132.1) had significant autoimmune kidney disease (glomerulonephritis) in addition to AIHA and ITP requiring renal transplantation. Patient #11.1 had acquired factor VIII inhibitor, hematuria, joint effusions and needed long term immunosuppression including weekly methotrexate. She is currently off all immunosuppressive medications and without any clinically significant autoimmune problems. More details of the clinical spectrum seen in this cohort of patients are presented in Table 1.
During follow up we noted significant hypogammaglobulinemia requiring IVIG associated with infiltrative lung lesions and arthropathies in two siblings from family #171. Their unique evolving clinical phenotype led to diagnosis of a second genetic defect (STAT3 gain of function defect: 2141C>T; p.P714L) in them. The Caspase 10 mutation seen in this family(pI406L) was also noted following EXOME sequencing in another child with congenital neurological findings and her healthy parent as well as healthy sibling. Hence we are attributing the more recent clinical findings of hypogammaglobulinemia, diffuse lymphocytic lung disease and pulmonary fibrosis in patients 171.1.and 171.4 to the recently described STAT3 GOF mutation (Reference). Lesson learnt from this one family we wish to share is that if evolving clinical phenotype in a patient does not fit the working diagnosis of a known monogenic disorder, it is worthwhile to expand the search for other coexisting genetic defects using extended genetic testing methods currently in vogue.
Reference:
Milner JD, Vogel TP, Forbes L, Ma CA, Stray-Pedersen A, Niemela JE, Lyons JJ, Engelhardt KR, Zhang Y, Topcagic N, Roberson ED, Matthews H, Verbsky JW, Dasu T, Vargas-Hernandez A, Varghese N, McClain KL, Karam LB, Nahmod K, Makedonas G, Mace EM, Sorte HS, Perminow G, Rao VK, O'Connell MP, Price S, Su HC, Butrick M, McElwee J, Hughes J, Willet J, Swan D, Xu Y, Santibanez-Koref M, Slowik V, Dinwiddie DL, Ciaccio CE, Saunders CJ, Septer S, Kingsmore SF, White AJ, Cant AJ, Hambleton S, Cooper MA. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2014 Oct 30.PMID: 25359994.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal