Abstract
Background: Hereditary stomatocytosis is an inherited disorder of the erythrocyte membrane responsible of chronic hemolytic anemia. Recent advances in the understanding of this group of diseases came from the identification of the molecular basis of this disorder. Mutations in the SLC4A1, FAM38A, RHAG, and SLC2A1 genes have been shown to cause different subtypes of hereditary stomatocytosis. Dehydrated hereditary stomatocytosis (DHSt) is due to mutations in the FAM38A gene coding for the mechanotransduction protein PIEZO1 and to the newly discovered mutations in the KCNN4 gene encoding the Gardos channel. It is important to recognize this entity and differentiate it from hereditary spherocytosis as patients with HSt develop severe and sometimes lethal thromboembolic complications following splenectomy. Also, some patients develop progressive and severe iron overload (IO) despite well compensated hemolysis and no or little transfusion requirement. It is unclear why patients have such different clinical features regarding hemolytic anemia and IO. We describe herein the impact of inherited and acquired modifiers of iron status on the phenotypic expression of DHSt.
Patients & Methods: We describe four patients (3 related and 1 unrelated) with proven DHSt due to FAM38A mutations, who displayed varying degrees of iron load.
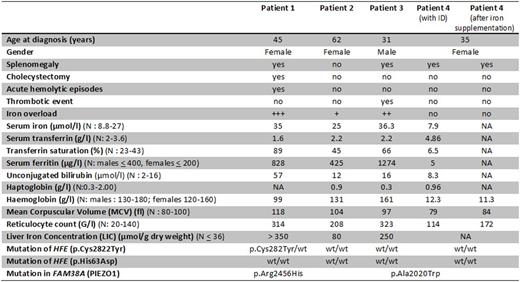
Results: The four reported patients were referred to our specialized outpatient consultation (center of expertise on rare iron overload) for investigation. Their clinical, laboratory and radiological features are summarized in the Table. It is noteworthy that both index cases were initially referred for investigation of hyperferritinemia. Iron levels closely correlated with the degree of hemolysis and with the severity of the clinical complications. One female patient with severe iron overload suffered from chronic anemia, acute hemolytic episodes, and symptomatic gallstones requiring cholecystectomy while one male patient with severe iron overload suffered from a thrombotic event. The two other female patients with no or moderate iron overload had no or mild hemolysis. Genetic modifiers increasing iron stores, such as the presence of the HFE C282Y mutation, and possibly the gender (male), were accompanied with higher liver iron concentration, increased hemolysis and clinical manifestations. On the opposite, females with normal or low iron stores (iron deficiency anaemia (ID) due to gynecologic bleedings) displayed no or mild hemolytic manifestations. It is noteworthy that in the female with ID no clinical or biological manifestations of hemolysis and of stomatocytosis were found initially (normal specialized phenotypic tests). The diagnosis was made by genetic analyses. Restoration of the iron stores resulted in the appearance of biological signs of hemolysis.
Conclusion: Iron overload or iron deficiency dramatically alter the clinical presentation of DHST due to PIEZO1 defects. The search for genetic or acquired causes of iron overload (or deficiency) is an important step in the evaluation of the clinical prognosis and the modulation of iron store may help in the management of the patients.
Clinical, biological, and radiological characteristics of the 4 patients
N: normal value; NA: not available; wt: wild-type; ID: iron deficiency
Clinical, biological, and radiological characteristics of the 4 patients
N: normal value; NA: not available; wt: wild-type; ID: iron deficiency
Cartron:Roche: Consultancy, Honoraria; Celgene: Honoraria; Gilead: Honoraria; Jansen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal