Abstract
Erythroferrone (ERFE) is a hormone produced by erythroblasts in the bone marrow in response to erythropoietin (EPO). Recent animal studies have shown that rather than being involved in regulation of baseline erythropoiesis, ERFE acts as a stress erythropoiesis-specific regulator of hepcidin expression. By suppressing hepcidin expression in the liver, EFRE contributes to increased dietary iron absorption and recycling of stored iron necessary for recovery of blood mass after hemorrhage. In addition, ERFE was found to be involved in hepcidin regulation in inherited iron loading anemias, such as b-Thalassemia. ERFE has potential as a clinical marker for assessing erythropoiesis in patients with blood disorders.
To date, there have been no reports of a human ERFE assay development and validation. To study the biological function and potential clinical applications of ERFE in humans, we developed the first dual monoclonal sandwich ELISA for serum measurement. Purified recombinant ERFE was used as antigen to immunize mice and subsequently screen 3000 hybridomas for binding to human ERFE. The nine positive hybridomas were expanded and monoclonal antibody from each clone purified and isotyped. We biotinylated each antibody and queried all possible combinations of capture and detection antibodies for binding activity. We discovered at least two pairs of antibodies suitable for assay optimization. Two mAbs were selected, 4C1 and 2D2, as the capture and detection antibody, respectively. We used streptavidin-HRP to quantify binding and detection of ERFE. The ERFE ELISA standard curve ranges from 0-50 ng/mL. The assay's lower limit of detection (LLOD) is 0.15 ng/mL and lower limit of quantitation (LLOQ) is 0.17 ng/mL. We assessed the normal range of ERFE by analyzing serum from110 healthy first time blood donors with normal iron status determined by assessment of ferritin, plasma iron, and transferrin saturation. Serum ERFE in the first time blood donors ranged from 0.15 to 3.94 ng/mL with a mean of 0.83 ng/mL.
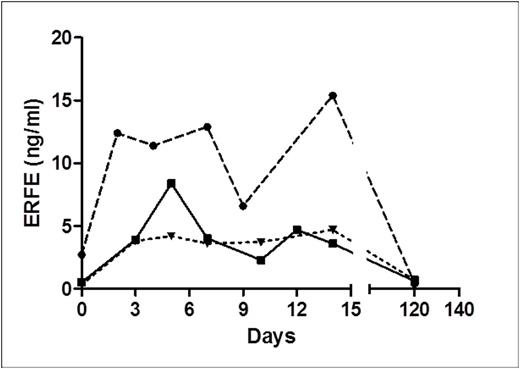
To re-capitulate the animal data previously observed in mice (Kautz et al., Nat Genet. 2014; 46(7): 678-684), we examined the effect of blood donation on human serum ERFE concentrations (Figure 1). We analyzed sera from three donors which underwent platelet and plasma-apheresis at baseline and day 2, 3, 4, 5, 7, 9, 10, 14, and 120 (Li et al., J Clin Apher., 2016). It was estimated that 30ml of RBCs were lost in the procedure. Serum ERFE concentrations were elevated from baseline in each patient within 2 days and remained higher through 14 days. At 120 days serum ERFE returned to baseline levels.
We went on to test the concept that serum ERFE concentrations would be elevated in blood disorders associated with ineffective erythropoiesis, we obtained serum samples from X-linked sideroblastic anemia probands and 15 of their family members. Nine of the probands had point mutation in the ALAS2 gene and two had a-globin duplications. We measured serum ERFE in the probands and family controls and discovered that ERFE was significantly increased in probands relative to familial controls (Figure 2). Family members had ERFE concentrations similar to healthy first time blood donors (<1 ng/ml).
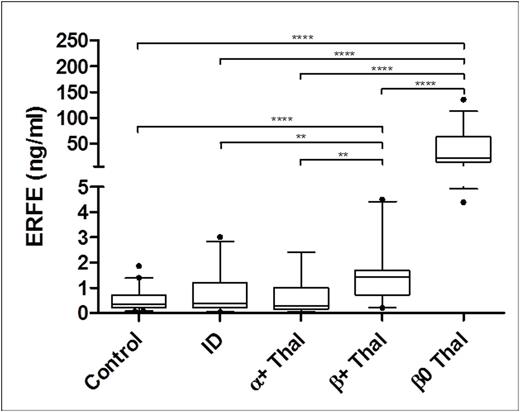
An additional study was conducted to examine ERFE in thalassemia patients whom are known to exhibit ineffective erythropoiesis due to mutations in the α- or β-globin genes that cause production of deformed red blood cells. We obtained sera from patients with both α- and β-thalassemia and discovered that both β+-thalassemia and β0-thalassemia patients had significantly higher serum ERFE concentrations than controls and a group of iron deficient (ID) controls (Figure 3). The β0-thalassemia patients had highly elevated serum ERFE which is likely due to the degree that erythropoiesis is affected.
The data we present lends strong support to the quality of the dual monoclonal sandwich ELISA we have developed. The ERFE assay is very sensitive, has excellent reproducibility and spike recovery characteristics, and is easy to perform. The physiological and clinical data we present supports the assertion that the assay is specific for ERFE and will allow insight into a number of hematological diseases.
Effect of plasma- or platelet-apheresis on ERFE.
ERFE in X-linked Sideroblastic Anemia. ****p<0.0001
ERFE in Iron Deficient and Thalassemic Patients. ****p<0.0001, **p<0.005
ERFE in Iron Deficient and Thalassemic Patients. ****p<0.0001, **p<0.005
Han:Intrinsic Lifesciences.: Employment, Equity Ownership. Westerman:Intrinsic LifeSciences: Employment. Ostland:Intrinsic LifeScienc s: Employment, Equity Ownership. Gutschow:Intrinsic LifeSciences: Employment, Equity Ownership. Olbina:Intrinsic LifeSciences: Employment, Equity Ownership. Westerman:Intrinsic LifeSciences: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal