Abstract
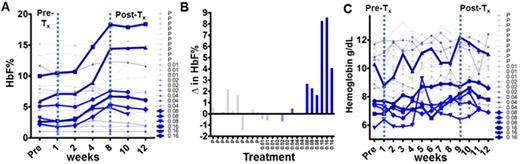
Fetal hemoglobin (HbF) potently corrects red cell pathology caused by β-globin (HBB) mutations in β-hemoglobinopathies (e.g., sickle cell disease, SCD). DNA methyltransferase (DNMT1) is a central component of the chromatin-modifying enzyme network that represses the gene for HbF, ƴ-globin (HBG), in adult erythropoiesis. Thus, DNMT1 has been scientifically validated as a molecular target to increase HbF. The generic deoxycytidine analogue decitabine (Dec) can incorporate into DNA without terminating chain elongation (the sugar in Dec is natural) and deplete DNMT1 without cytotoxicity. Unfortunately, Dec is very rapidly metabolized by cytidine deaminase (CDA) in vivo into uridine counterparts that do not deplete DNMT1. Moreover, the uridine degradation products can mis-incorporate into DNA and cause cytotoxicity. Also, solid tissues, e.g., intestine/liver, highly express CDA, severely limiting Dec tissue distribution, oral bioavailability and exposure time. To address these barriers to clinical translation, which have as a common cause CDA, we evaluated the combination of oral Dec with a CDA-inhibitor, tetrahydrouridine (THU). Pre-clinical in vivo studies indicated improved distribution and oral bioavailability (~10-fold increase) with the low Cmax but extended Tmax desired for non-cytotoxic DNMT1-depletion. Here we report the first-in-man clinical translation (NCT01685515). Patients: This Phase 1 clinical trial enrolled adult SS or S-b-thal patients with multiple, clinically significant complications of SCD and risk of early death despite standard of care hydroxyurea. The primary objective was to identify the dose of oral Dec that can be safely co-administered 2X/week with oral THU. Treatment: Fixed dose oral THU 10mg/kg was ingested 60 minutes before oral Dec at 0.01, 0.02, 0.04, 0.08 or 0.16 mg/kg (mpk) in 5 cohorts of 5 pts, randomized 3/2 THU-Dec/placebo (Fig.1). Repeat dose administration 2X/wk for 8 wks was used to assess safety, increasing the likelihood that the identified dose would be safe for the intended application of chronic disease modification. Pharmacokinetics: Samples were obtained at 0, 2, 4 and 24 hrs from 12 of 15 THU-Dec subjects (dictated by venous access). Dec was detected (standard LCMS/MS method) at the lowest dose 0.01 mpk, and a dose-dependent increase was observed. Dec 0.08 and 0.16 mpk produced Cmax 9-21 and 39-54 nM respectively, levels that produced clinical pharmacodynamic effects (described below). Adverse events (AE):AE were similar in placebo and THU-Dec cohorts. No significant AE, including no significant gastro-intestinal AE, were attributed to study drug. There were no Grade 4 AE. No subjects discontinued placebo or THU-Dec. Hematology: HbF was measured by HPLC and in individual red cells by flow cytometry (F-cells). Dec 0.08 and 0.16 mpk substantially increased HbF (~2-fold increase in F-cells at 0.16 mpk) (Fig.1). In parallel, total hemoglobin (hb) increased (e.g., by 1.9, 1.8 and 1.2 g/dL at 0.16mpk) while LDH, t.bilirubin and D-dimers decreased (improvement in hemolysis and thrombophilia biomarkers) (Fig.1). Non-cytotoxic DNMT1-depletion alters hematopoietic differentiation and is expected to simultaneously increase platelet counts and decrease absolute neutrophil counts. This was seen at Dec 0.08 and 0.16 mpk. The HbF and Hb increases and biomarker improvements were produced within boundaries of platelet and neutrophil counts that did not require treatment holds. Discussion: Combining oral Dec with oral THU to inhibit CDA thus produced the Dec tissue distribution and wide concentration-time profile that is a basic requirement for clinical translation of observations regarding the safety and value of DNMT1 as a molecular target for therapy, moreover with the accessibility of oral administration. Platelet count increases by non-cytotoxic DNMT1-depletion were not linked with increases in thrombophilia biomarkers here or in previous trials of Dec in SCD. This impression is consistent also with clinical experience after standard of care splenectomy, which increases platelet counts even more drastically. Multi-organ damage in SCD incorporates the bone marrow, causing a decline in reticulocytosis/hb that contributes to early death. This underscores the import in further evaluation of this non-cytotoxic, normal stem cell sparing, rationally targeted approach to disease modification of SCD.
Lavelle:Acetylon: Research Funding. Hsu:Gerson Lehman Group: Consultancy; Mast Therapeutics: Research Funding; Eli Lilly: Research Funding; Astra Zeneca: Consultancy, Research Funding; Centers for Medicare and Medicaid Innovation: Research Funding; EMMI Solutions: Consultancy; Purdue Pharma: Research Funding; Hilton Publishing: Consultancy, Research Funding; Sancilio: Research Funding; Pfizer: Consultancy, Research Funding. DeSimone:EpiDestiny: Consultancy, Other: patents around decitabine and tetrahydrouridine. Saunthararajah:EpiDestiny: Consultancy, Other: patents around decitabine and tetrahydrouridine.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal