Abstract
Introduction: The BTK inhibitor ibrutinib (IB) is highly active in WM, achieving major responses (CR+VGPR+PR) in approximately 70% of pts. However, VGPR is infrequent, with rates ≤15% in reported series (Treon NEJM 2015, Dimopoulos EHA 2016). BGB-3111 is a potent, highly-specific and irreversible BTK inhibitor, with greater selectivity than IB for BTK vs. other TEC- and HER-family kinases, and superior bioavailability. We previously reported that the recommended phase 2 dose of BGB-3111 is 320mg daily (in single or split dose) in pts with advanced B cell malignancies. This achieves plasma levels equivalent to 6-10 fold that of IB, and >90% continuous inhibition of BTK in lymph node biopsies. We specifically investigated the safety and efficacy of BGB-3111 in pts with WM in an expansion cohort of the initial Phase 1 trial. Reported here are updated results of this study, including data from WM specific expansion cohorts.
Aims: To define the safety profile, and clinical activity of BGB-3111 in pts with WM.
Methods: These results are from a pre-specified a component of a Phase 1 study (Part 1: dose escalation [DEsc] in pts with R/R B cell malignancies, Part 2: disease-specific dose expansion cohorts [EC] at the recommended Phase 2 dose that included patients with relapsed / refractory or previously untreated WM). Adverse events (AEs) were reported per CTCAE v4.03. Responses were determined according to the modified Sixth International Workshop on WM (IWWM) criteria. The data cut-off is 10 June 2016.
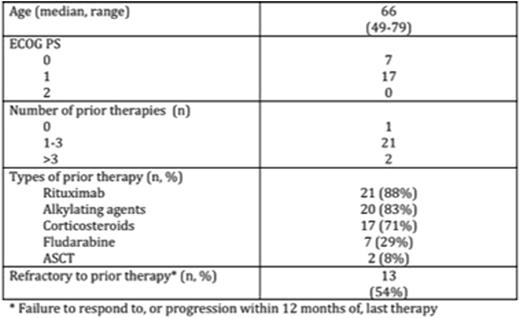
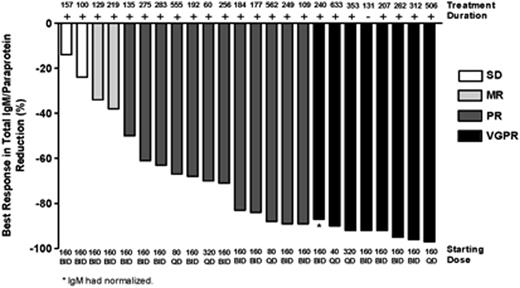
Results: As of 10 June 2016, 31 pts with WM have been enrolled; 6 pts in DEsc (40mg [n=2], 80mg [n=2], 160mg QD [n=1], and 320mg QD [n=1]), and 25 in the WM EC (160mg BID [n=18], 320mg QD [n=7]). Three pts in DEsc were increased to 160mg QD after analysis of DEsc data, as allowed by protocol. Twenty-four pts are included in this analysis; 5 pts were excluded because of short (<60 day) follow-up for safety and efficacy, and 2 pts accrued at a single site were excluded because of insufficient documentation at baseline. Patient demographics, disease characteristics, and prior treatment history are summarized in Table 1. BGB-3111 was well tolerated with 71% reporting no drug related AE >Gr 1 severity within the first 12 weeks of therapy. The most frequent AEs (>20%) of any attribution (all Gr 1/2) were upper respiratory infection (25%), diarrhea (25%), and nausea (21%). There were 2 SAEs assessed as possibly related to BGB-3111 (Gr 2 atrial fibrillation, Gr 3 cryptococcal meningitis); in both cases, BGB-3111 was temporarily held but safely resumed. In total, 2 pts developed AF (one Gr 1, one Gr 2). No serious hemorrhage (≥Gr 3 or CNS hemorrhage of any grade) was reported. After a median follow-up of 7.6 months (2-21 months), the response rate was 92% (22/24). The major response rate was 83% (20/24), with VGPR (>90% reduction in IgM and reduction in extramedullary disease) in 33% (8/24) and PR (50-90% reduction in IgM and reduction in extramedullary disease) in 50% (12/24) pts. Pts with WM refractory to their last therapy were equally responsive: major response 77% [10/13], VGPR 31% [4/13], PR 46% [6/13]. Median time to initial response and major response were 29 days and 34 days, respectively. IgM decreased from a median of 29.9g/l at baseline to 3.0g/l; hemoglobin increased from a median of 10.1 g/dl at baseline to 13.5g/l. Two of 3 pts who had a dose increase after reaching a stable IgM plateau had further falls in IgM levels after dose escalation. Kinetics of IgM and hemoglobin response are presented in Figure 1. Lymphadenopathy was present in 8 pts at baseline; serial CT demonstrated reduction or resolution in all 8 pts (27%-100% reduction in SPD). Only one patient discontinued BGB-3111, due to exacerbation of pre-existing bronchiectasis while in VGPR. There have been no cases of disease progression. Analysis of response by genomic characteristics (including MYD88 and CXCR4 mutational status) is ongoing.
Conclusions: BGB-3111 is well-tolerated and highly active in WM. The depth and quality of responses, as reflected by the VGPR rate of 33%, warrant a randomized comparison against ibrutinib in pts with WM.
Tam:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; janssen: Honoraria, Research Funding. Opat:Roche: Consultancy, Honoraria, Other: Provision of subsidised drugs, Research Funding. Simpson:Amgen Pharmaceuticals: Research Funding; Celgene, Roche, Janssen: Honoraria. Anderson:Walter and Eliza Hall Institute of Medical Research: Other: Walter and Eliza Hall Institute of Medical Research receives milestone payments for the development of venetoclax. Kirschbaum:Beigene: Employment. Wang:Beigene: Employment. Xue:Beigene: Employment. Yang:BeiGene: Employment. Hedrick:Beigene: Employment. Seymour:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Roberts:AbbVie: Research Funding; Servier: Research Funding; Genentech: Research Funding; Janssen: Research Funding; Genentech: Patents & Royalties: Employee of Walter and Eliza Hall Institute of Medical Research which receives milestone payments related to venetoclax.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal