Abstract
Background: Effective therapy for classical hairy cell leukemia (c-HCL) refractory to purine nucleoside analog (PNA) treatment is limited, and there are no accepted treatment standards for variant hairy cell leukemia (v-HCL). Ibrutinib, an oral small molecule inhibitor of Bruton tyrosine kinase (BTK), has shown single-agent efficacy and acceptable tolerability in patients with various B-cell malignancies, including chronic lymphocytic leukemia, mantle cell lymphoma, and Waldenstrom's. While BTK is expressed in HCL cells, the clinical activity of ibrutinib in this disease has not been previously assessed. We conducted a single-arm, multi-center phase 2 study (NCI #9268) of single-agent ibrutinib to characterize the overall response rate (complete + partial response) and safety of single-agent ibrutinib treatment for HCL.
Methods: Patients with c-HCL (unfit for or relapsed after PNA) and v-HCL (relapsed or treatment-naïve) were eligible. Enrolled patients required treatment, had ECOG ≤ 2, no active infection, and preserved end-organ function. Patients received continuous once daily ibrutinib in 28-day cycles. Using a Simon 2-stage design, the first 13 patients were treated at 420 mg daily, and a second cohort of 13 patients was accrued at the 840 mg dose-level. Ultimately, the pre-specified number of objective responses was reached at the 420 mg dose-level, and accrual to the second stage of the design continues at that dose. Response, including bone marrow biopsy with immunohistochemistry for minimal residual disease (MRD), was assessed after 8 and 12 cycles. Patients experiencing clinical benefit may continue ibrutinib until unacceptable toxicity or progressive disease.
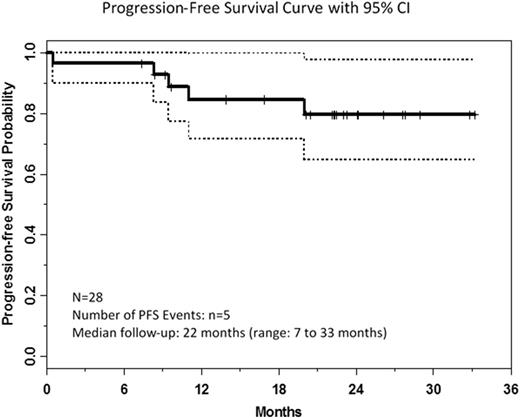
Results: As of 15 May 2016, 28 patients had been enrolled and were evaluable for response: 420 mg/day (n=15) and 840 mg/day (n=13). Enrolled patients (1 treatment-naïve v-HCL, 10 relapsed v-HCL, 17 relapsed c-HCL) included 22 male and 6 female patients with median age 65 years (range: 43-78). Relapsed patients received median 4 prior therapies (range: 1-11); all had prior PNA, 81% had prior rituximab, and 3 c-HCL patients had prior vemurafenib. Response data are summarized by dose and histology in the table. To date, the ORR is 46% [4 CR (all c-HCL) and 9 PR (6 c-HCL)], with objective responses more commonly observed in patients with c-HCL. Eight additional patients (29%) with stable disease have experienced clinical benefit (resolution of symptoms, improvement in peripheral blood cell counts) not meeting criteria for PR, most commonly attributable to persistent thrombocytopenia, and continue on treatment. At median follow-up of 22 months, 20 patients (71%) remain on treatment, 3 patients (1 v-HCL, 2 c-HCL) have progressed, 2 patients (c-HCL) with severe neutropenia at baseline (c-HCL) had early deaths from pneumonia, 1 (c-HCL) discontinued during cycle 8 for failure to resolve baseline neutropenia, and 2 other patients (v-HCL) have discontinued for adverse events. Estimated 24-month progression-free survival (PFS) was 79% (95% CI: 57-91%) and the median PFS has not been reached. Redistribution lymphocytosis was observed in patients with circulating disease at baseline. Soluble interleukin-2 receptor (sIL2R) level was increased at baseline in all c-HCL patients for whom data were available and correlated with response. In general, toxicity did not differ by dose level or disease histology. The most frequent (>10%) grade ≥3 adverse events (AEs) were lymphopenia (21%), neutropenia (18%), lung infection (18%), thrombocytopenia (14%), hypophosphatemia (11%), and hypertension (11%). Common grade 1/2 non-hematologic toxicities included myalgia (57%), diarrhea (54%), fatigue (50%), nausea/vomiting (46%), bruising (46%), and rash (46%); grade 1/2 atrial fibrillation (no grade ≥3) was observed in 5 patients. Grade ≥3 infection was observed in 25%, but no grade ≥3 bleeding AEs were reported.
Conclusions:Ibrutinib can induce remission in both c-HCL and v-HCL patients, including heavily pre-treated patients, but complete remissions have only been observed in c-HCL to date. The drug is generally well-tolerated during long-term administration, and durable clinical benefit is observed in the majority of treated patients even when objective response criteria are not met.
Jones:Pharmacyclics, LLC, an AbbVie Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Lozanski:Stemline Therapeutics Inc.: Research Funding; Beckman Coulter: Research Funding; Genentech: Research Funding; Boehringer Ingelheim: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal