Abstract
Background: Rearrangement of chromosome 11q23 at KMT2A (MLL+) occurs in ~20% of childhood acute myeloid leukemia (AML) cases and is the most common recurrent cytogenetic abnormality in childhood AML. More than 80 fusion partners of MLL have been characterized and the prognostic impact varies by partner and additional cytogenetic aberrations (ACAs). We previously reported superior event-free-survival (EFS), overall survival (OS), relapse risk (RR) and disease free survival (DFS) for patients with MLL+ on AAML0531 treated with gemtuzumab ozogamicin (GO). The impact of fusion partners has not been described for this trial cohort.
Objective: To determine the impact of 11q23/MLL+ fusion partners on outcomes for children with AML who were treated uniformly on AAML0531.
Methods: AAML0531 (2006 - 2010) was a randomized phase 3 trial of GO with conventional chemotherapy for children, adolescents, and young adults, ages 0 to 29 years, with de novo AML. The trial enrolled 1022 eligible patients without Down syndrome, and cytogenetic data was available for 988 patients. Of those, 215 (21.8%) were classified as 11q23/MLL+ after central cytogenetic review. The majority of patients with 11q23/MLL+ AML (n=200, 93%) were classified as intermediate risk (IR) and a minority (n=15, 7%) were classified as high risk (HR) based upon high FLT3 internal tandem duplication allelic ratio (FLT3-ITD-AR), monosomy 5/5q deletion, monosomy 7, or >15% blasts by morphology following Induction (Ind) I. HSCT was performed for 30 patients (14%).
We defined 11q23/MLL+ subgroups for partners identified in ≥5 cases. Partners in <5 cases were grouped as "other"; unidentified fusions were classified as "unknown". Outcomes of minimal residual disease (MRD) at end of Ind I and complete remission (CR) at end of Ind II, 5-yr EFS and OS from study entry, and 5-yr RR from end of Ind II for patients in CR were compared across subgroups. Univariable and multivariable analyses were performed.
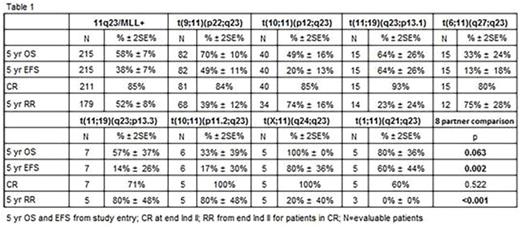
Results: The median age for the 11q23/MLL+ cohort was 2.5 years and the distribution was equally male and female. The majority of cases (n=175, 81%) were classified into 8 specific partner subgroups, and the remaining cases were classified as other (n=30, 14%) or unknown (n=10, 5%). The most common translocation was t(9;11)(p22;q23) (n= 82, 38%). Central nervous system involvement was rare (<10% overall and in every subgroup). While extramedullary chloromas were rare (7% overall), chloromas were reported in 83% (n=5) of t(10;11)(p11.2;q23), 40% (n=16) of t(10;11)(p12;q23), and 27% (n=4) of t(6;11)(q27;q23). ACAs were observed in 86 of 211 cases (41%) that underwent central review. ACAs were numerical in 54 cases (26%), most commonly trisomy 8 (n=30, 14%) and structural + numerical in 32 cases (15%), most commonly involving chromosome 1 (n=11, 34%). Molecular findings for 3 studied genes were rare: High FLT3-ITD-AR n=4; NPM1 n=1; CEBPA n=0.
Clinical outcomes varied significantly by fusion partner and poorer OS was primarily due to relapse (Table 1). CR rate was 86% overall and did not vary by subgroups. MRD was detected in 21% overall and was most common in t(1;11)(q21;q23) and t(6;11)(q27;q23). Complex karyotype (≥3 abnormalities) was seen in 52 cases (25%) and predicted inferior outcome compared to <3 abnormalities (5-yr EFS 29% vs. 41%, p=0.02; 5-yr OS 44% vs. 63%, p=0.001, respectively). Chromosome 1 abnormalities were associated with very poor outcomes, 5-yr EFS 19% and 5-yr OS 29%. The most unfavorable partners for EFS were t(6;11)(q27;q23), t(10;11)(p11.2;q23), t(10;11)(p12;q23), and t(11;19)(q23;p13.3)(Figure 1). In multivariable comparison to patients with t(9;11)(p22;q23), the former 3 groups maintained inferior EFS (HR 2.51, p=0.006; HR 3.10, p<0.001; HR 2.15, p=0.002, respectively) and OS (HR 2.56, p=0.017; HR 4.8, p=0.006; HR 2.14, p=0.016, respectively).
Conclusions: Fusion partners critically impact the prognosis of 11q23/MLL+ AML. In this large, uniformly treated cohort, t(6;11)(q27;q23), t(10;11)(p11.2;q23) and t(10;11)(p12;q23) were independently associated with inferior EFS and OS. Children with these unfavorable partners are at high risk for relapse and allogeneic HSCT should be considered in first CR. Complex karyotype, chromosome 1 aberrations, and extramedullary chloromas were additional adverse prognostic factors. Work is ongoing to confirm these results in the subsequent COG trial, AAML1031.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal