Abstract
Background:
The past 30 years has heralded significant improvements in the treatment and outcomes of patients with advanced-stage Hodgkin lymphoma, with the introduction of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) and various combinations of BEACOPPbaseline and BEACOPPescalated (bleomycin,etoposide, doxorubicin, cyclophosphamide, vincristine,procarbazine, and prednisone). Initially, BEACOPP-containing regimens demonstrated superior progression-free survival (PFS) and overall survival (OS) compared to ABVD, despite higher rates of hematologic toxicity, infections, and infertility. However, the superiority of this regimen was tempered by concerns of increased toxicity and long-term complications including secondary malignancy and infertility. Furthermore, quality-adjusted measures and patient preferences have not been factored into analyses of the evidence. We performed a decision analysis to explore the trade-off that occurs when initial improvements in progression-free survival and overall survival are balanced with increased morbidity and mortality associated with infections, secondary malignancies, and infertility in the BEACOPP-containing strategy.
Methods:
We developed a Markov decision-analytic model to compare ABVD versus BEACOPPbaseline and BEACOPPescalated (hereinafter referred to as BEACOPP) for a hypothetical cohort of transplant-eligible patients withnewly-diagnosed, advanced-stage Hodgkin lymphoma. The model simulates the clinical course of patients over a 20-year time horizon, with the end-points of life expectancy and quality-adjusted life expectancy. The baseline probabilities used in the model were derived from a systematic review of published randomized controlled trials. Key variables included response, relapse and survival rates comparing ABVD versus BEACOPP, risk of developing complications such as infection, infertility, or secondary malignancy with each strategy, and the estimated survival once secondary malignancy develops. We also incorporated therapies for relapsed disease including autologous stem cell transplantation, and post-transplant strategies, based on available data. Efficacy was discounted by 3%. The model incorporated data on health state utilities, which were derived from a review of the literature. Sensitivity analyses were performed for key variables.
Results:
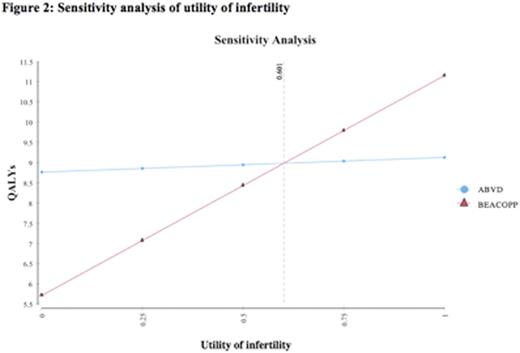
Based on a 20-year model with 100,000 trials, life expectancy was 10.9 years with ABVD and 12.3 years with BEACOPP, resulting in a net benefit of 1.4 years for the BEACOPP strategy. The quality-adjusted life expectancies for the two strategies, respectively, were 9.1 and 10.5 years, with an expected benefit of 1.4 QALYs with BEACOPP. Sensitivity analyses demonstrated that the model was robust to the key variables of probability of death from secondary malignancy, probability of relapse, and probability of infertility secondary to BEACOPP. A range of relapse probabilities post-ABVD and BEACOPP were tested in sensitivity analyses, and BEACOPP was consistently superior, with the lowest difference between QALYs found to be 0.5 QALYs. In sensitivity analysis of treatment-related mortality secondary to BEACOPP, the threshold value was found to be 8% mortality over the6 monthtreatment period, a value much greater than that reported in the literature (see Figure 1). The threshold utility of infertility was found to be 0.60 in sensitivity analysis (see Figure 2), a value lower than the utility derived from a systemic review of the literature (0.87). Onmicrosimulation(100,000 trials), 88% of the simulations showed that BEACOPP was the preferred strategy compared to ABVD.
Conclusion:
The preferred treatment strategy for patients with newly diagnosed advanced-stage Hodgkin lymphoma is a combination BEACOPP regimen. This strategy maximizes life expectancy and quality-adjusted life years, accounting for the increased rates of hematologic toxicity, secondary malignancy, and infertility in patients receiving the BEACOPP strategy. The model was robust to sensitivity analyses of key variables tested through plausible ranges obtained from the published literature.
Buckstein:Novartis: Honoraria; Celgene: Honoraria, Research Funding. Prica:Celgene: Honoraria; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal