Abstract
Venous thromboembolism (VTE) is an unpredictable and life-threatening toxicity that occurs early in acute lymphoblastic leukemia (ALL) therapy. The incidence is approximately 5% in children diagnosed with ALL [Caruso et al. Blood. 2006;108(7):2216-22], which is higher than in other pediatric cancer types [Athale et al. Pediatric Blood & Cancer. 2008;51(6):792-7]. Clinical risk factors for VTE in children during ALL therapy include older age and the use of asparaginase. We hypothesized that there may be additional risk factors that can modify VTE risk, beyond those previously reported [Mitchell et al. Blood. 2010;115(24):4999-5004]. We sought to define early predictive clinical factors that could select a group of children at highest risk of VTE, with possible utility in an interventional trial of prophylactic anticoagulation.
We conducted a retrospective study of 1021 Australian children, aged 1-18 years, treated between 1998-2013 on successive BFM-based ALL therapies. Patient records were reviewed to ascertain incidence of VTE; and to systematically document clinical variables present at diagnosis and during induction/consolidation phases of therapy. The CTCAE v4.03 system was used for grading of VTE events. Multivariate logistic and cox regression were used to determine significant clinical risk factors associated with VTE (SPSS v23.0). All P values were 2-tailed, significance level <.05.
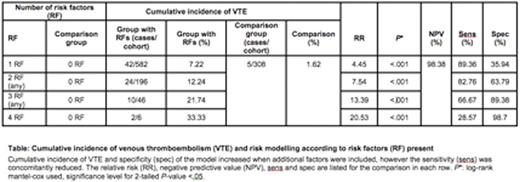
The incidence of on-treatment VTE was 5.09% [96% ≥Grade 2 (CTCAE v4.0)]. Age ≥10 years [P =.048, HR 1.96 (95% confidence interval= 1.01-3.82)], positive blood culture in induction/consolidation [ P =.009, HR 2.35 (1.24-4.46)], extreme weight at diagnosis <5th or >95th centile [ P =.028, HR 2.14 (1.09-4.20)] and elevated peak gamma-glutamyl transferase (GGT) >5 x upper limit normal in induction/consolidation [ P =.018, HR 2.24 (1.15-4.36)] were significantly associated with VTE in multivariate cox regression modeling. The cumulative incidence of VTE, if all 4 clinical risk factors in our model were present, was 33.33%, which is significantly greater than the incidence of VTE for a patient without any risk factors (1.62%, P <.001).
These 4 clinical factors could be used as a basis for assigning thromboprophylaxis in children with ALL. Our model detected 80% (42/52) of all VTE events by incorporating one or more risk factors. An equal proportion of patients eventually developing VTE could be predicted by weight and age ≥10 years; or later bacteremia and elevated GGT. Bacteremia, when present as a risk factor, preceded VTE in 80% of cases (20/25 cases) at a median of 29 days before VTE (range 3-668 days).
The negative predictive value (NPV), specificity and sensitivity for the 4 risk factor model were 98.38%, 98.70% and 28.57% respectively.
If 3 specified risk factors were included in the algorithm, such as 2 baseline and one treatment-related variable, the incidence of VTE was ≥25%, NPV 98.38%, specificity ≥96.19% and sensitivity 80%. The high NPV and high specificity mean the model can successfully exclude children who are not at increased risk of VTE. The challenge is to balance unnecessary exposure to anticoagulation against the risk of development of VTE.
We have identified novel clinical risk factors in induction/consolidation - positive blood culture, hepatic enzymatic elevation and extreme weight at diagnosis- that may highlight risk mechanisms related to VTE pathogenesis. Our predictive model can define a group at highest risk of VTE who may benefit from randomized trials of prophylactic anticoagulation in childhood ALL therapy.
Acknowledgments: The authors acknowledge support from the Kids Cancer Alliance (a Translational Cancer Research Centre of Cancer Institute NSW), Cancer Institute New South Wales, Royal Australasian College of Physicians - Kids Cancer Project Research Entry Scholarship, Cancer Therapeutics CRC (CTx) PhD Clinician Research Top-Up Scholarship, The Kids Cancer Project, Australian and New Zealand Children's Haematology Oncology Group, ASSET study members, data managers and clinical research associates at each site.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal