Abstract
Background: BCMA is expressed on MM cells, and CAR T cells targeting BCMA have pre-clinical anti-MM activity. CART-BCMA is an autologous T cell product engineered by lentiviral transduction to express a fully human BCMA-specific CAR with CD3ζ and 4-1BB signaling domains, and then expanded ex vivo using CD3/CD28 beads.
Methods: In this ongoing, 3+3 dose-escalation study, relapsed/refractory MM patients (pts) receive CART-BCMA cells as split-dose infusions (10% on day 0, 30% on day 1, and 60% on day 2). Three cohorts are planned: 1) 1-5 x 108 CART cells alone; 2) cyclophosphamide (CTX) 1.5 g/m2 + 1-5 x 107 CART cells; and 3) CTX 1.5 g/m2 + 1-5 x 108 CART cells. Pts need serum creatinine (Cr) <2.5 mg/dL or Cr clearance≥30 ml/min, and adequate hepatic, cardiac, and pulmonary function. BCMA expression on MM cells is analyzed by flow cytometry, though no pre-specified level is required for eligibility. CART-BCMA frequency and activation status are assessed in blood and marrow by flow cytometry. Levels of CAR-transduced cells are also measured by qPCR using a transgene-specific primer/probe pair. Soluble BCMA, BAFF and APRIL levels in serum are assessed by ELISA. Bioactivity of the infusion product and CART-related cytokine release syndrome are analyzed by Luminex. Responses are assessed by IMWG criteria.
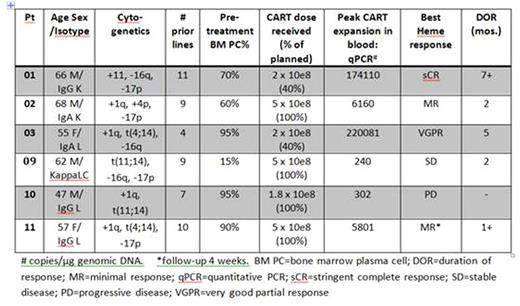
Results: To date, 11 pts have been screened, and 6 treated in cohort 1. Reasons for not receiving treatment were screen fail (n=2), rapid MM progression/renal failure (n=2), and pt/MD choice (n=1). The 6 treated pts were all IMID/PI-refractory with high risk cytogenetics and median 9 lines of therapy (Table). All expressed BCMA on MM cells, and achieved the minimum target dose of 1x108 CART-BCMA cells. All but 2 received 100% of planned dose, with 2 (pts 01and 03) receiving 40% (3rd infusions held for fever). Cytokine release syndrome (CRS) occurred in 5 patients: 2 grade 3 requiring tocilizumab (pts 01 and 03), 1 grade 2, and 2 grade 1. High-grade CRS was associated with elevated levels of IL-6, IFNg, MCP1, MIG, IL2Ra, and IL-10, as seen in our acute lymphoblastic leukemia CTL019 trial (Teachey et al, 2016). There was 1 DLT: grade 4 PRES (posterior reversible encephalopathy syndrome) in pt 03, with severe delirium, recurrent seizures, obtundation, and cerebral edema on MRI. This resolved after anti-epileptics, high-dose methylprednisolone and cyclophosphamide, without long-term neurologic sequelae. Other grade 3/4 toxicities to date include hypophosphatemia (n=3 pts), hypocalcemia (n=2), and anemia, neutropenia, lymphopenia, thrombocytopenia, hypofibrinogenemia, fatigue, pneumonia, UTI, elevated Alk phos and AST, hypokalemia, hypertension, and pleural effusion (n=1 each). CART-BCMA cells were detected in blood and marrow by CAR-specific PCR in all 6 pts, and in 4/6 by flow cytometry, with 2 pts, 01 and 03, having massive CART expansion peaking at 90% and 76% of peripheral CD3+ T cells, respectively. CART-BCMA cells during peak expansion were predominantly CD8+ and highly activated. Pt 01 has ongoing CART-BCMA persistence, with ongoing stringent CR at 7 months and MRD-negative bone marrow by flow cytometry. Pt 03, who had pleural and possible dural MM involvement, had CART-BCMA cells found in pleural fluid and CSF, and achieved VGPR (IF+ only) with resolution of extramedullary disease on PET/CT scan. She progressed at 5 months, associated with significant reduction of CART-BCMA cells and loss of BCMA expression on her MM cells by flow cytometry, suggestive of antigen escape. Two pts (02, 11) had modest CART-BCMA expansion, with 1 minimal response (MR) lasting 2 months, and 1 ongoing MR 1 month post-infusion. Two pts (09, 10) had minimal expansion and no response. Soluble BCMA levels, which were elevated in all pts at baseline, declined in parallel with CART-BCMA expansion and correlated with depth of response, with an accompanying increase in previously suppressed BAFF and APRIL levels in serum.
Conclusions: CART-BCMA cells can be manufactured from heavily-pretreated MM pts, and demonstrate promising in vivo expansion and clinical activity, even without lymphodepleting conditioning. Depth of response correlates with degree of CART-BCMA expansion and CRS. Toxicities to date include CRS and in 1 pt, severe reversible neurotoxicity, as described in other CAR T cell studies. Expanded accrual in cohort 1, as well as in cohorts with CTX conditioning, is ongoing, with updated data to be presented at the meeting.
Cohen:Bristol-Meyers Squibb: Consultancy, Research Funding; Janssen: Consultancy. Garfall:Bioinvent: Research Funding; Novartis: Consultancy, Research Funding; Medimmune: Consultancy. Stadtmauer:Novartis: Consultancy; Takada: Consultancy; Amgen: Consultancy; Celgene: Consultancy; Teva: Consultancy; Janssen: Consultancy. Lacey:Novartis: Research Funding. Lancaster:Janssen: Consultancy; Medimmune, Inc.: Consultancy; Grifols, Inc.: Other: Teaching courses. Vogl:Millennium: Consultancy, Research Funding; Celgene: Consultancy; Karyopharm: Consultancy; Teva: Consultancy; Acetylon: Research Funding; Glaxo Smith Kline: Research Funding; Calithera: Research Funding; Constellation: Research Funding. Ambrose:Novartis: Research Funding. Plesa:Novartis: Patents & Royalties, Research Funding. Kulikovskaya:Novartis: Research Funding. Weiss:Prothena: Other: Travel, accommodations, Research Funding; Novartis: Consultancy; GlaxoSmithKline: Consultancy; Janssen: Consultancy, Other: Travel, accommodations, Research Funding; Millennium: Consultancy, Other: Travel, accommodations. Richardson:Novartis: Employment, Patents & Royalties, Research Funding. Isaacs:Novartis: Employment. Melenhorst:Novartis: Patents & Royalties, Research Funding. Levine:Novartis: Patents & Royalties, Research Funding. June:Novartis: Honoraria, Patents & Royalties: Immunology, Research Funding; University of Pennsylvania: Patents & Royalties; Tmunity: Equity Ownership, Other: Founder, stockholder ; Johnson & Johnson: Research Funding; Celldex: Consultancy, Equity Ownership; Immune Design: Consultancy, Equity Ownership; Pfizer: Honoraria. Milone:Novartis: Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal