Abstract
Background:
Momelotinib is a JAK 1/2 inhibitor that is active in the treatment of myelofibrosis (MF); a previous phase 1/2 study included 100 consecutive patients from the Mayo Clinic (Leukemia. 2015;29:741). In the current long-term study of these 100 patients, we provide a cumulative account of short and long term efficacy and toxicity data, as well as survival analysis.
Methods:
The current study represents sponsor-independent analysis. The study patients are part of a larger (n=166) phase-1/2 momelotinib study (NCT00935987), which was conducted in two dose-escalation (100 mg-400 mg daily doses) and dose-confirmation (300 mg daily dose) phases. Adverse events (AEs) were monitored by Common Terminology Criteria for Adverse Events (Version 4.03) and responses by the International Working Group criteria (Blood. 2013;122:1395). All statistical analyses considered clinical and laboratory parameters obtained at the time of entry to study.
Results:
Baseline data: 100 patients with MF (median age 66 years; 58% males) were treated between 11/20/09 and 11/10/10; 64 patients had primary MF, 22 post-polycythemia vera MF and 14 post-essential thrombocythemia MF. 73 (73%) patients harbored JAK2 mutations, 16 (16%) CALR, 7 MPL and 4 were "triple-negative"; among the 16 CALR-mutated cases, 13 were classified as "type 1/type 1-like". DIPSS-plus risk distribution (JCO 2011;29:392) was 63% high, 36% intermediate-2 and 1% intermediate-1; 49% displayed red cell transfusion-dependency, 58% constitutional symptoms, 87% palpable splenomegaly >5 cm and 50% abnormal karyotype. 94 patients were screened for ASXL1 mutations with 41 (44%) mutated and 78 for SRSF2 mutations with 14 (18%) mutated. 21 (21%) patients were previously treated with another JAK inhibitor.
Current disposition: All information was updated in July 2016. To-date, after a median follow-up of 3.2 years, 88 drug discontinuations, 70 deaths and 14 leukemic transformations have been documented; median follow-up for living patients was 5.7 years (range 5.1-6.4). Among the 30 patients who are currently alive, 12 remain on study and another 5 have received stem cell transplant.
Toxicity data: After a median treatment duration of 1.7 years, "momelotinib related" grade 3 or 4 AEs included thrombocytopenia (34%), neutropenia (9%), anemia (5%), increased lipase (7%), increased ALT (4%), increased AST (2%), increased ALP (2%) and headaches (2%). In addition, noteworthy grade 1 or 2 AEs included peripheral neuropathy (PN) 47%, increased lipase (14%), increased amylase (17%), increased bilirubin (13%), increased AST (21%), increased ALT (19%), increased APTT (17%), headaches (13%), dizziness (22%), nausea (23%) and diarrhea (20%). Most of the AEs, except PN, were reversible.
Efficacy data and predictors of response and relapse-free survival: Clinical improvement (CI) was documented in 57% of patients, anemia response in 44%, and spleen response in 43%. 51% of transfusion-dependent patients became transfusion independent. The majority of patients also had marked improvement in their symptoms. 46 (81%) of the 57 responding patients discontinued treatment after a median treatment duration of 2.3 years. ASXL1 mutations predicted inferior CI (p=0.03) whereas relapse-free survival was adversely affected by absence of type 1/type 1-like CALR (p=0.03) or presence of unfavorable karyotype (p=0.002); among the 11 responding patients currently still receiving the drug, all had favorable karyotype and 5 had type 1/type 1-like CALR mutations.
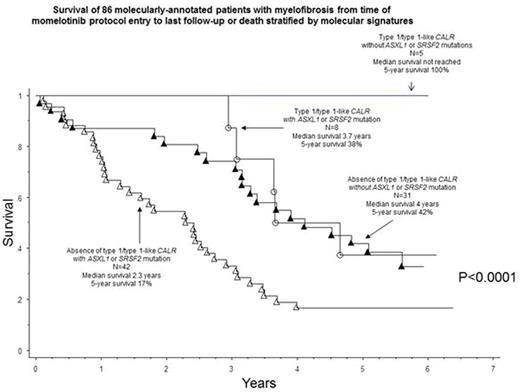
Survival analysis and risk factors: Median survival, calculated from the time of study entry, was 3.2 years with 5-year survival rate of 32%. In multivariable analysis of genetic markers, absence of type 1/type 1-like CALR (HR 2.9, 95% CI 1.1-7.2) or presence of SRSF2 (2.9, 1.5-5.4) or ASXL1 (1.8, 1.1-3.2) mutations were independently predictive of shortened survival (Figure).
Conclusions:
Momelotinib therapy in MF provides effective palliation in terms of anemia, splenomegaly and constitutional symptoms. However, less than 20% of treated patients enjoy durable long-term benefit and almost half experience drug-related and mostly irreversible peripheral neuropathy. Long-term survival and durability of response were superior in patients with type 1/type 1-like CALR mutations and inferior in those with ASXL1/SRSF2 mutations or unfavorable karyotype.
Al-Kali:Onconova Therapeutics, Inc.: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal