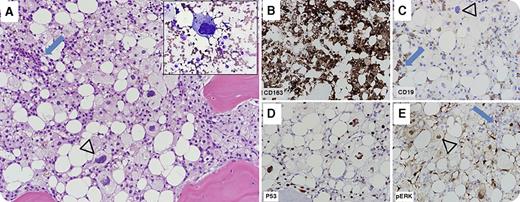
A 22-year-old woman diagnosed with Philadelphia chromosome-negative B-acute lymphoblastic leukemia (B-ALL) was treated with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone therapy. She achieved complete remission and was maintained on 6-mercaptopurine, vincristine, methotrexate, and prednisone therapy. She relapsed 14 months later and was reinduced. She presented to our hospital shortly thereafter, at which time bone marrow evaluation showed persistent B-ALL (blue arrows) admixed with an extensive infiltrate (arrowheads) of intermediate to large neoplastic cells with foamy cytoplasm, pleomorphic nuclei, and prominent nucleoli (panel A, original magnification ×20; hematoxylin and eosin stain; inset, Wright-Giemsa stain; inset and all other panels, original magnification ×40), positive for the histiocytic/monocytic marker CD163 (panel B) and negative for CD19 (panel C). Next-generation mutation analysis showed NRAS (c.35G>A/p.G12D) and TP53 (c.839G>T/p.R280I) mutations; these were associated with p53 accumulation (panel D) and phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) overexpression (panel E) in the histiocytic sarcoma cells. 18-Fluorodeoxyglucose–positron emission tomography/computed tomography (18FDG-PET/CT) showed widespread disease. The patient was treated with ifosfamide, carboplatin, and etoposide followed by clofarabine, idarubicin, plus cytarabine salvage therapy. Subsequent bone marrow evaluation and 18FDG-PET/CT imaging showed resolution of prior findings. The patient died from disseminated Fusarium infection during preparation for allogeneic stem cell transplant.
Histiocytic sarcoma is a rare aggressive neoplasm that may arise as a secondary malignancy, occasionally as a transdifferentiation phenomenon, in conjunction with B-ALL and other lymphoid malignancies.
A 22-year-old woman diagnosed with Philadelphia chromosome-negative B-acute lymphoblastic leukemia (B-ALL) was treated with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone therapy. She achieved complete remission and was maintained on 6-mercaptopurine, vincristine, methotrexate, and prednisone therapy. She relapsed 14 months later and was reinduced. She presented to our hospital shortly thereafter, at which time bone marrow evaluation showed persistent B-ALL (blue arrows) admixed with an extensive infiltrate (arrowheads) of intermediate to large neoplastic cells with foamy cytoplasm, pleomorphic nuclei, and prominent nucleoli (panel A, original magnification ×20; hematoxylin and eosin stain; inset, Wright-Giemsa stain; inset and all other panels, original magnification ×40), positive for the histiocytic/monocytic marker CD163 (panel B) and negative for CD19 (panel C). Next-generation mutation analysis showed NRAS (c.35G>A/p.G12D) and TP53 (c.839G>T/p.R280I) mutations; these were associated with p53 accumulation (panel D) and phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) overexpression (panel E) in the histiocytic sarcoma cells. 18-Fluorodeoxyglucose–positron emission tomography/computed tomography (18FDG-PET/CT) showed widespread disease. The patient was treated with ifosfamide, carboplatin, and etoposide followed by clofarabine, idarubicin, plus cytarabine salvage therapy. Subsequent bone marrow evaluation and 18FDG-PET/CT imaging showed resolution of prior findings. The patient died from disseminated Fusarium infection during preparation for allogeneic stem cell transplant.
Histiocytic sarcoma is a rare aggressive neoplasm that may arise as a secondary malignancy, occasionally as a transdifferentiation phenomenon, in conjunction with B-ALL and other lymphoid malignancies.
For additional images, visit the ASH IMAGE BANK, a reference and teaching tool that is continually updated with new atlas and case study images. For more information visit http://imagebank.hematology.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal