Key Points
The combination of ixazomib and dexamethasone has clinical activity in patients with relapsed and or refractory multiple myeloma.
Higher dose of ixazomib leads to improved response rates but with higher rates of treatment related adverse events.
Abstract
Proteasome inhibitors have become an integral part of myeloma therapy. Considerable efforts have gone into optimizing this therapeutic approach to obtain maximal proteasome inhibition with least toxicity. Ixazomib is the first oral proteasome inhibitor to enter the clinic and has been studied as a single agent as well as in various combinations. The current trial was designed to examine the efficacy and toxicity of combining 2 different doses of ixazomib (4 mg and 5.5 mg given weekly for 3 of 4 weeks) with 40 mg weekly of dexamethasone, in relapsed myeloma. Seventy patients were enrolled, 35 patients randomly assigned to each ixazomib dose. Overall, 30 (43%; 95% confidence interval, 31-55) of the patients achieved a confirmed partial response or better, with 31% achieving a response with 4 mg and 54% with 5.5 mg of ixazomib. The median event-free survival (EFS) for the entire study population was 8.4 months; 1-year overall survival was 96%. The EFS was 5.7 months for patients with prior bortezomib exposure and 11.0 months for bortezomib-naïve patients. A grade 3 or 4 adverse event considered at least possibly related to treatment was seen in 11 (32%) patients at 4 mg and in 21 (60%) at 5.5 mg. Dose reductions were more frequent with 5.5 mg dose. Overall, the ixazomib with dexamethasone has good efficacy in relapsed myeloma, is well-tolerated and with higher response rate at 5.5 mg, albeit with more toxicity. This study was registered at www.clinicaltrials.gov as #NCT01415882.
Introduction
Proteasome inhibitors (PIs) are integral components of currently used regimens, both for treatment of newly diagnosed and relapsed multiple myeloma (MM), and particularly in patients with certain cytogenetic abnormalities such as t(4;14) that is associated with aggressive disease behavior.1,2 Bortezomib, the initial PI to enter the clinic, has shown considerable efficacy in all stages of MM therapy.3-6 PIs, when combined with immunomodulatory drugs (IMiDs) such as lenalidomide or alkylating agents, have resulted in some of the most effective treatment regimens in MM to date.7-9 In a meta-analysis of randomized clinical trials of initial therapy of MM, the use of bortezomib was associated with better overall survival (OS).10 However, it is associated with high risk of peripheral neuropathy and also requires weekly clinic visits for parenteral administration. Although the risk of peripheral neuropathy with bortezomib has been mitigated to some extent with the weekly schedule and the use of subcutaneous administration, it still remains a concern.11,12 Carfilzomib, a second-generation proteasome inhibitor, has significantly lower risk of peripheral neuropathy compared with bortezomib and has been shown to have improved efficacy compared with bortezomib in a phase 3 trial, but requires twice-weekly IV infusion.13,14 As the proteasome inhibition continues to be optimized in the clinic, the ability to administer this class of drugs orally can have a profound effect on the convenience as well as the ability to provide prolonged therapy. Even though the optimal duration of therapy remains unknown, continuous therapy until disease progression is increasingly being incorporated into the initial treatment paradigm in MM, and the availability of an oral PI will allow us to maximize the efficacy of this class of drugs in management of MM.
Ixazomib citrate (MLN9708) is the first orally bioavailable inhibitor of the 20S proteasome to be evaluated for treatment of MM.15 Ixazomib citrate is a modified peptide boronic acid and is the citrate ester of ixazomib (MLN2238), the biologically active moiety. Ixazomib citrate rapidly hydrolyzes to ixazomib upon contact with aqueous solution or plasma. Ixazomib preferentially binds the β5 site of the 20S proteasome at lower doses, with inhibition of the β1 and β2 sites at higher concentrations. Compared with bortezomib, nonclinical studies have shown that ixazomib has a faster dissociation rate from the proteasome. Ixazomib has demonstrated antitumor activity in a range of tumor xenograft models, including MM models.16,17 In clinical trials, ixazomib has shown promising activity as a single agent in patients with relapsed and refractory MM, with very low rates of peripheral neuropathy observed in the single-agent trials.18-20 Ixazomib has shown considerable efficacy in combination with immunomodulatory drugs in newly diagnosed and relapsed, refractory MM. It was recently approved by Food and Drug Administration for use in relapsed MM, based on the results of a phase 3 trial demonstrating improved progression-free survival (PFS) when added to lenalidomide and dexamethasone.21 However, given the older age spectrum of patients with MM, many are unable to tolerate multidrug combinations, and the doublets of a novel agent with dexamethasone continues to have a role in the treatment of this disease, as demonstrated by the positive results from the randomized trial of lenalidomide and dexamethasone in elderly patients.22 The cost of the drugs also often limit the number of drugs that can be used in combinations. Given that the majority of patients in the early trials with ixazomib had previously been exposed to bortezomib, we designed this trial to better understand the efficacy of ixazomib in combination with dexamethasone in patients with relapsed MM with limited exposure to bortezomib. Furthermore, given that the maximum tolerated dose of ixazomib in the phase 1 trials was 5.5 mg and the 4 mg dose was explored in the combination trials, we also wanted to examine the efficacy of each of these doses in combination with dexamethasone.
Patients and methods
Study design
This open-label, randomized, phase 2 study evaluated the safety, tolerability, and efficacy of 2 different doses of oral ixazomib citrate (arm B = 4 mg and arm C = 5.5 mg) given weekly along with dexamethasone in patients with relapsed MM, who either had no exposure to PIs or had limited (no more than 6 cycles) exposure to a bortezomib-containing regimen. The study enrolled patients from February 2013 to April 2015. It was performed in accordance with the provisions of the Declaration of Helsinki, the International Conference on Harmonization, and the Guidelines for Good Clinical Practice, and with approval of the Mayo Clinic Institutional Review Board. The results from arm A, which was a phase 2 study of ixazomib with addition of dexamethasone for lack of response or disease progression, was previously reported.23
Study objectives
The primary objective of the study was to determine the confirmed overall response rate (≥ partial response[PR]) of 2 different doses of ixazomib with dexamethasone, in patients with relapsed MM, who were proteasome inhibitor–naïve or had received fewer than 6 cycles of therapy with bortezomib or carfilzomib, and were not refractory (defined as progressing on therapy or within 60 days of stopping therapy) to bortezomib. The secondary objectives included assessment of event-free survival (EFS) and overall survival (OS) following treatment with ixazomib with dexamethasone. The study was not designed to formally compare the 2 dose levels in terms of efficacy or toxicity.
Patient selection
The study enrolled patients, 18 years of age or older, with MM that had relapsed after at least 1 previous therapy. Patients were required to have measurable disease (serum M-protein ≥1 g/dL or urine M-protein ≥200 mg/24 hours or involved free light chain level ≥10 mg/dL, provided the serum free light chain ratio was abnormal), Eastern Cooperative Oncology Group performance status of 0 to 2, adequate hematologic (absolute neutrophil count ≥1000/mm3, platelets ≥75 000/mm3), hepatic (total bilirubin ≤1.5 × upper limit of normal, alanine/aspartate aminotransferase ≤3 × upper limit of normal), and renal (creatinine clearance ≥30 mL/minute) function. Patients with grade ≥3 peripheral neuropathy or grade 2 with pain, grade >1 diarrhea, or who had major surgery or serious infection within 14 days before start of therapy were excluded. Patients receiving systemic treatment with strong CYP1A2 inhibitors or strong inhibitors/inducers of CYP3A within 14 days were excluded. Other factors that precluded participation in the trial included uncontrolled cardiovascular conditions (including uncontrolled hypertension, uncontrolled cardiac arrhythmias, symptomatic congestive heart failure, unstable angina, or myocardial infarction within the past 6 months); known HIV positivity, known hepatitis B surface antigen–positive status, or known or suspected active hepatitis C infection; and known allergy to any of the study medications, their analogs, or excipients in the various formulations. Other comorbidities or severe preexisting illness that in the treating physician’s opinion could interfere with oral absorption and/or tolerance of ixazomib citrate excluded patients from participation.
Drug administration
Patients were randomly assigned to receive ixazomib orally at a dose of 4 mg or 5.5 mg on days 1, 8, and 15 of a 28-day cycle. On both arms, dexamethasone at a dose of 20 mg orally was given on days 1, 2, 8, 9, 15, and 16 of the 28-day cycle. Patients were allowed to remain on therapy until disease progression, unacceptable toxicity, patient preference, or physician discretion. Dose modifications were made for ixazomib-related toxicities with successive reductions in its dose to 4 mg (if assigned to the 5.5-mg arm), 3 mg, and 2 mg followed by discontinuation if the 2-mg dose was not tolerated. Prophylactic antiemetics were recommended before each dose of ixazomib. Prophylactic antidiarrheals were not used; however, the administration of antidiarrheals was allowed after infectious causes were excluded. Topical steroids and other symptomatic measures were permitted for management of any skin rash. All patients received herpes zoster prophylaxis.
Assessments
Adverse events (AEs) were graded using the National Cancer Institute’s Common Terminology Criteria for AEs, version 4.0. Myeloma disease response was done in accordance with the International Myeloma Working Group uniform criteria, incorporating the additional category of minor response (MR). All response categories required confirmation of the required tests with the exception of the bone marrow used for complete response (CR) determination. At any point in treatment, patients suspected of progressive disease (PD) had response assessments repeated to confirm disease progression.
Statistical analyses
The primary end point in each arm of this study was the overall response rate (ORR) with ixazomib and dexamethasone, where a success was defined as stringent complete response (sCR), complete response (CR), very good partial response (VGPR), or partial response (PR) noted as the objective status on 2 consecutive evaluations. All patients meeting the eligibility criteria, who signed a consent form, and received at least 1 dose of the drug were evaluable for response, with the exception of patients who were determined to be a major treatment violation. Patients were randomized between the 2 arms using a dynamic allocation procedure, with stratification factors including previous bortezomib or carfilzomib exposure (none vs limited) and whether the patient was lenalidomide refractory (yes vs no). The same 1-stage binomial design was used in each arm independently. With 32 evaluable patients randomized to each arm, the study provided 88% power to test the null hypothesis that the ORR is at most 20% vs the alternative hypothesis that the ORR is at least 40%, with a 1-sided significance level of α = 0.09 (independently for each arm). An additional 3 patients were enrolled on each arm to account for ineligible patients and protocol violations, for a total of 70 patients across the entire study. For toxicity assessment, all patients who received at least 1 dose of study drug were included in the analysis. OS was defined as the time from study entry to death due to any cause. Event-free survival (EFS) was defined as the time from study entry to disease progression, death from any cause, or subsequent treatment of myeloma. Duration of response (DOR) was defined as the time from when the patient’s objective status is first noted to be ≥PR to the date progression is documented in patients who achieved a response. The distributions of time to event measures were estimated using the Kaplan-Meier method.
Results
Patients
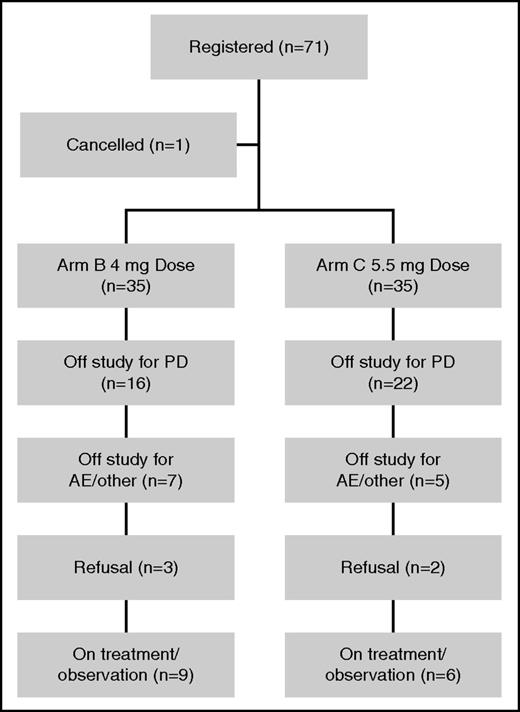
Seventy-one patients were enrolled; 1 patient was considered ineligible and excluded from all analyses. The median age for the 70 evaluable patients was 70 years (range, 46-84) and 53% were male. The baseline characteristics at study entry for each arm are described in Table 1. Prior therapies included IMiDs (90%), bortezomib (30%), and autologous stem cell transplantation (71%). Across the entire study, at the time of data cutoff, 44 (63%) patients had progressed and 61 (87%) were alive, with a median follow-up of 16.3 months (range, 0.9-30.7). Forty-five (64%) patients have received subsequent treatment of myeloma, in which 2 patients received alternate treatment before documented disease progression. Fifteen patients remain on therapy; reasons for drug discontinuation were disease progression (38), patient refusal (5), AE (5), alternate treatment (6), and physician discretion (1). Patient disposition by study arm is outlined in Figure 1.
Baseline characteristics
| . | Arm B (4 mg) (N = 35) . | Arm C (5.5 mg) (N = 35) . |
|---|---|---|
| Age, median (range) | 71.0 (46.0-83.0) | 69.0 (51.0-84.0) |
| Male, N (%) | 16 (45.7) | 21 (60.0) |
| ECOG performance score, N (%) | ||
| 0 | 26 (74.3) | 28 (80.0) |
| 1 or 2 | 9 (25.7) | 7 (20.0) |
| Months from diagnosis to on study, median (range) | 65.4 (17.8-268.4) | 45.8 (7.2-175.8) |
| Cytogenetic risk | ||
| High (del 17p, t(4;14), t(14;16)) | 5 (14.3%) | 5 (14.3%) |
| Standard | 30 (85.7%) | 30 (85.7%) |
| Number of prior planned regimens, median (range) | 4 (2-8) | 4 (2-5) |
| Prior therapies, N (%) | ||
| Alkylators | 25 (71.4) | 27 (77.1) |
| Thalidomide | 5 (15.6) | 6 (19.4) |
| Lenalidomide | 27 (84.4) | 28 (90.3) |
| Pomalidomide | 5 (15.6) | 5 (16.1) |
| Bortezomib | 9 (25.7) | 12 (34.3) |
| Previous transplant, N (%) | 24 (68.6) | 26 (74.3) |
| Refractory to lenalidomide, N (%) | 16 (45.7) | 16 (45.7) |
| Bortezomib or carfilzomib exposure, limited, N (%) | 11 (31.4) | 12 (34.3) |
| . | Arm B (4 mg) (N = 35) . | Arm C (5.5 mg) (N = 35) . |
|---|---|---|
| Age, median (range) | 71.0 (46.0-83.0) | 69.0 (51.0-84.0) |
| Male, N (%) | 16 (45.7) | 21 (60.0) |
| ECOG performance score, N (%) | ||
| 0 | 26 (74.3) | 28 (80.0) |
| 1 or 2 | 9 (25.7) | 7 (20.0) |
| Months from diagnosis to on study, median (range) | 65.4 (17.8-268.4) | 45.8 (7.2-175.8) |
| Cytogenetic risk | ||
| High (del 17p, t(4;14), t(14;16)) | 5 (14.3%) | 5 (14.3%) |
| Standard | 30 (85.7%) | 30 (85.7%) |
| Number of prior planned regimens, median (range) | 4 (2-8) | 4 (2-5) |
| Prior therapies, N (%) | ||
| Alkylators | 25 (71.4) | 27 (77.1) |
| Thalidomide | 5 (15.6) | 6 (19.4) |
| Lenalidomide | 27 (84.4) | 28 (90.3) |
| Pomalidomide | 5 (15.6) | 5 (16.1) |
| Bortezomib | 9 (25.7) | 12 (34.3) |
| Previous transplant, N (%) | 24 (68.6) | 26 (74.3) |
| Refractory to lenalidomide, N (%) | 16 (45.7) | 16 (45.7) |
| Bortezomib or carfilzomib exposure, limited, N (%) | 11 (31.4) | 12 (34.3) |
ECOG, Eastern Cooperative Oncology Group.
Patient disposition across the entire study for both treatment arms.
Patient disposition across the entire study for both treatment arms.
Response to therapy and survival
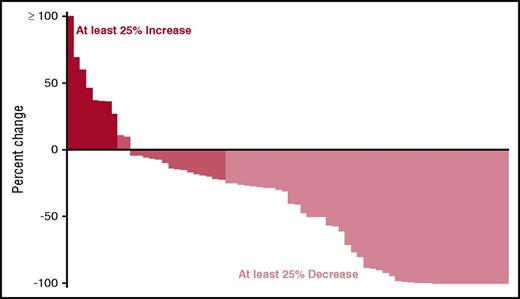
Overall, 30 (43%; 95% confidence interval [CI], 31-55) of the patients achieved a confirmed PR or better across the entire trial, with 31% (95% CI, 17-49) achieving a response in arm B and 54% (95% CI, 37-71) in arm C. An additional 6 patients achieved a minor response, 5 in arm B and 1 in arm C; translating to a clinical benefit rate (CBR) of 51% (95% CI, 39-64). The responses by treatment arm are detailed in Table 2. Among patients receiving the higher dose of ixazomib, the response rates were similar irrespective of the prior exposure to bortezomib, but were lower for those with prior exposure to bortezomib in patients receiving the lower dose of ixazomib. The response rates grouped by refractoriness to lenalidomide, bortezomib sensitivity, and fluorescence in situ hybridization–based risk status are shown in Table 3. A waterfall plot highlighting the depth of the responses observed is shown in Figure 2. Responses occurred rapidly, with the median time to response being 1.1 months (range, 0.8-3.6) in the low-dose arm and 1.0 month (range, 0.8-13.0) in the high-dose arm.
Efficacy outcomes and follow-up status
| . | Arm B (4 mg) (N = 35) . | Arm C (5.5 mg) (N = 35) . |
|---|---|---|
| ORR | 31% (95% CI, 17-49) | 54% (95% CI, 37-71) |
| No. of responders | 11 | 19 |
| sCR | 0 | 1 |
| CR | 1 | 0 |
| VGPR | 7 | 10 |
| PR | 3 | 8 |
| MR | 5 | 1 |
| Median OS* | NA | NA |
| % alive at 6 mo | 100 | 100 |
| Median EFS* | 8.4 mo (95% CI, 4.5-12.8) | 7.8 mo (95% CI, 4.6-11.9) |
| %Event free at 6 mo | 60% (95% CI, 46-79) | 60% (95% CI, 46-79) |
| Median duration of response | 16.7 mo (95% CI, 9.3-NA) | 16.3 mo (95% CI, 8.6-NA) |
| Median time to response, mo (range) | 1.1 (0.8-3.6) | 1.0 (0.8-13.0) |
| Patients with progression, N (%) | 19 (54.0) | 25 (71.4) |
| Patients alive, N (%) | 30 (85.7) | 31 (88.6) |
| Median follow-up (alive patients), mo (range) | 16.2 (0.9-30.7) | 16.3 (2.0-28.7) |
| Last cycle administered | 8 (1-27) | 7 (1-31) |
| Patients with new treatment | 22 (63%) | 23 (66%) |
| Before disease progression | 2 | 0 |
| On treatment | 9 | 6 |
| Reason for ending treatment, N (%) | ||
| Refused further treatment | 3 (11.5) | 2 (6.9) |
| AE | 2 (7.7) | 3 (10.3) |
| Disease progression | 16 (61.5) | 22 (75.9) |
| Alternate treatment | 4 (15.4) | 2 (6.9) |
| Physician discretion | 1 (3.8) | 0 (0.0) |
| . | Arm B (4 mg) (N = 35) . | Arm C (5.5 mg) (N = 35) . |
|---|---|---|
| ORR | 31% (95% CI, 17-49) | 54% (95% CI, 37-71) |
| No. of responders | 11 | 19 |
| sCR | 0 | 1 |
| CR | 1 | 0 |
| VGPR | 7 | 10 |
| PR | 3 | 8 |
| MR | 5 | 1 |
| Median OS* | NA | NA |
| % alive at 6 mo | 100 | 100 |
| Median EFS* | 8.4 mo (95% CI, 4.5-12.8) | 7.8 mo (95% CI, 4.6-11.9) |
| %Event free at 6 mo | 60% (95% CI, 46-79) | 60% (95% CI, 46-79) |
| Median duration of response | 16.7 mo (95% CI, 9.3-NA) | 16.3 mo (95% CI, 8.6-NA) |
| Median time to response, mo (range) | 1.1 (0.8-3.6) | 1.0 (0.8-13.0) |
| Patients with progression, N (%) | 19 (54.0) | 25 (71.4) |
| Patients alive, N (%) | 30 (85.7) | 31 (88.6) |
| Median follow-up (alive patients), mo (range) | 16.2 (0.9-30.7) | 16.3 (2.0-28.7) |
| Last cycle administered | 8 (1-27) | 7 (1-31) |
| Patients with new treatment | 22 (63%) | 23 (66%) |
| Before disease progression | 2 | 0 |
| On treatment | 9 | 6 |
| Reason for ending treatment, N (%) | ||
| Refused further treatment | 3 (11.5) | 2 (6.9) |
| AE | 2 (7.7) | 3 (10.3) |
| Disease progression | 16 (61.5) | 22 (75.9) |
| Alternate treatment | 4 (15.4) | 2 (6.9) |
| Physician discretion | 1 (3.8) | 0 (0.0) |
NA, not attained.
Kaplan Meier.
Response rate (≥PR) to therapy based on disease characteristics
| . | Arm B (4 mg) . | Arm C (5.5 mg) . | Arm B (4 mg) . | Arm C (5.5 mg) . |
|---|---|---|---|---|
| . | Prior bortezomib . | No prior bortezomib . | ||
| Response rate | 1/9 (11%) | 6/12 (50%) | 10/26 (38%) | 13/23 (57%) |
| sCR | 0 | 0 | 0 | 1 |
| CR | 0 | 0 | 1 | 0 |
| VGPR | 1 | 4 | 6 | 6 |
| PR | 0 | 2 | 3 | 6 |
| MR | 0 | 0 | 5 | 1 |
| . | Arm B (4 mg) . | Arm C (5.5 mg) . | Arm B (4 mg) . | Arm C (5.5 mg) . |
|---|---|---|---|---|
| . | Prior bortezomib . | No prior bortezomib . | ||
| Response rate | 1/9 (11%) | 6/12 (50%) | 10/26 (38%) | 13/23 (57%) |
| sCR | 0 | 0 | 0 | 1 |
| CR | 0 | 0 | 1 | 0 |
| VGPR | 1 | 4 | 6 | 6 |
| PR | 0 | 2 | 3 | 6 |
| MR | 0 | 0 | 5 | 1 |
| . | Intermediate or high risk . | Standard risk . | ||
|---|---|---|---|---|
| Response rate | 1/5 (20%) | 3/5 (60%) | 10/30 (33%) | 16/30 (53%) |
| sCR | 0 | 0 | 0 | 1 |
| CR | 0 | 0 | 1 | 0 |
| VGPR | 1 | 1 | 6 | 9 |
| PR | 0 | 2 | 3 | 6 |
| MR | 1 | 0 | 4 | 1 |
| . | Intermediate or high risk . | Standard risk . | ||
|---|---|---|---|---|
| Response rate | 1/5 (20%) | 3/5 (60%) | 10/30 (33%) | 16/30 (53%) |
| sCR | 0 | 0 | 0 | 1 |
| CR | 0 | 0 | 1 | 0 |
| VGPR | 1 | 1 | 6 | 9 |
| PR | 0 | 2 | 3 | 6 |
| MR | 1 | 0 | 4 | 1 |
| . | Refractory to lenalidomide . | Not refractory to lenalidomide . | ||
|---|---|---|---|---|
| Response rate | 5/16 (31%) | 8/16 (50%) | 6/19 (32%) | 11/19 (58%) |
| sCR | 0 | 1 | 0 | 0 |
| CR | 1 | 0 | 0 | 0 |
| VGPR | 2 | 4 | 5 | 6 |
| PR | 2 | 3 | 1 | 5 |
| MR | 3 | 1 | 2 | 0 |
| . | Refractory to lenalidomide . | Not refractory to lenalidomide . | ||
|---|---|---|---|---|
| Response rate | 5/16 (31%) | 8/16 (50%) | 6/19 (32%) | 11/19 (58%) |
| sCR | 0 | 1 | 0 | 0 |
| CR | 1 | 0 | 0 | 0 |
| VGPR | 2 | 4 | 5 | 6 |
| PR | 2 | 3 | 1 | 5 |
| MR | 3 | 1 | 2 | 0 |
Waterfall plot showing the distribution of depth of response observed across both arms.
Waterfall plot showing the distribution of depth of response observed across both arms.
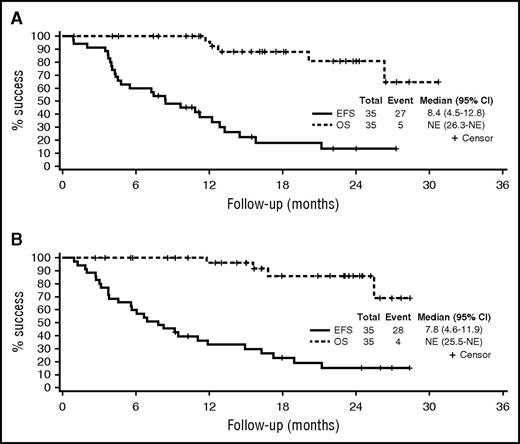
The median EFS for the entire study population was 8.4 months (95% CI, 4.5-12.8); 1-year OS was 96% (95% CI, 91-100; Table 2, Figure 3). Across the entire study, the EFS was 5.7 months (95% CI, 3.7-9.2) for patients with prior exposure to bortezomib compared with 11.0 months (95% CI, 6.0-14.5) for the bortezomib-naïve patients. The median DOR among the 30 patients with a PR or better was 16.7 months (95% CI, 13.1-not attained). The median EFS and the DOR were similar across the 2 arms and are detailed in Table 2.
OS and EFS for the each of the study arms. (A) Arm B. (B) Arm C. NE, not evaluable.
OS and EFS for the each of the study arms. (A) Arm B. (B) Arm C. NE, not evaluable.
Dose intensity and AEs
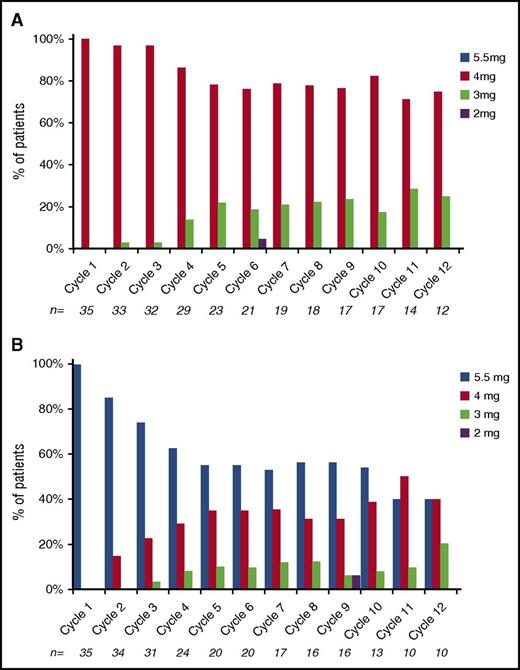
Patients received a median of 7 cycles of therapy (range, 1-31) across the trial; 53 (76%) and 34 (49%) patients received at least 4 and 8 cycles, respectively, and 21 (30%) patients stayed on trial for more than 12 cycles. The median number of cycles of treatment was similar for the 2 arms, 8 (range, 1-28) and 7 (range, 1-31) for the low- and high-dose arms respectively. The total number of cycles delivered was similar between the 2 arms, 347 cycles for the low-dose arm and 341 cycles for the high-dose arm. Dose modifications for ixazomib were required in a higher proportion of patients in the high-dose arm compared with the low-dose arm (43% vs 17%), whereas that for dexamethasone was similar (34% vs 23%) (Figure 5). In terms of delivered dose, 85% of the intended dose of ixazomib was delivered in the high-dose arm compared with 95% of the intended dose in the low-dose arm.
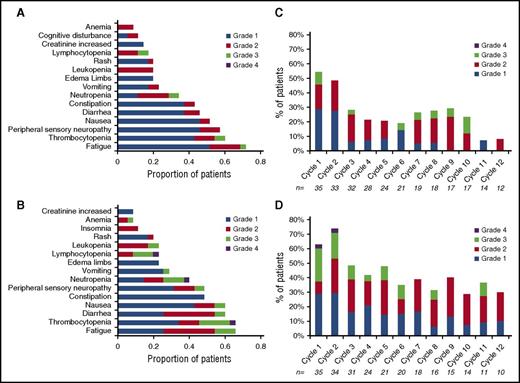
An AE of any grade that was considered at least possibly related was reported in 100% of the patients in both arms. There were no treatment-related deaths in either of the arms. A grade 3 or 4 AE considered at least possibly related to drug was seen in 9 (26%) and 2 (6%) patients in the low-dose arm and in 19 (54%) and 2 (6%) patients in the high-dose arm, respectively (Table 4). The most common grade 2 or higher AEs observed included fatigue, neutropenia, thrombocytopenia, and diarrhea. Peripheral neuropathy at least possibly related to the drug was seen in 33 patients (grade 1), 8 patients (grade 2), and 2 patients (grade 3; both in the high-dose arm). Figure 4A provides the distribution of all grades of the most common toxicities considered at least possibly related to the drug administration. No cumulative hematological toxicity was observed in either arm of the trial (Figure 4B). Five patients went off study because of an AE (2 in the low-dose arm, 3 in the high-dose arm); these included continued grade 2 neuropathy and heart failure.
Treatment administration, tolerability, and adverse events
| . | Arm B (4 mg) . | Arm C (5.5 mg) . |
|---|---|---|
| Ixazomib | ||
| Number of cycles | 347 | 341 |
| Median cycle total dose (range) | 12 (2-12) | 16.5 (4-16.5) |
| Number of patients with dose reductions, N (%) | 6 (17) | 15 (43) |
| Total number of dose reductions | 7 | 21 |
| Dexamethasone | ||
| Number of cycles | 345 | 325 |
| Median cycle total dose (range) | 120 (0-240) | 120 (0-160) |
| Number of patients with dose reductions, N (%) | 8 (23) | 12 (34) |
| Total number of dose reductions | 10 | 16 |
| Treatment delays | ||
| Number of patients, N (%) | 9 (26) | 10 (29) |
| Total number of delays | 12 | 12 |
| . | Arm B (4 mg) . | Arm C (5.5 mg) . |
|---|---|---|
| Ixazomib | ||
| Number of cycles | 347 | 341 |
| Median cycle total dose (range) | 12 (2-12) | 16.5 (4-16.5) |
| Number of patients with dose reductions, N (%) | 6 (17) | 15 (43) |
| Total number of dose reductions | 7 | 21 |
| Dexamethasone | ||
| Number of cycles | 345 | 325 |
| Median cycle total dose (range) | 120 (0-240) | 120 (0-160) |
| Number of patients with dose reductions, N (%) | 8 (23) | 12 (34) |
| Total number of dose reductions | 10 | 16 |
| Treatment delays | ||
| Number of patients, N (%) | 9 (26) | 10 (29) |
| Total number of delays | 12 | 12 |
| Frequency of AEs . | Regardless of attribution . | At least possibly related . | Regardless of attribution . | At least possibly related . |
|---|---|---|---|---|
| Evaluable, N (%) | 35 (100) | 35 (100) | 35 (100) | 35 (100) |
| Grade 3+, N (%) | 15 (43) | 9 (26) | 26 (74) | 19 (54) |
| Grade 4+, N (%) | 2 (6) | 2 (6) | 3 (9) | 2 (6) |
| Grade 3+ hematologic, N (%) | 6 (17) | 5 (14) | 15 (43) | 13 (37) |
| Grade 4+ hematologic, N (%) | 0 (0) | 0 (0) | 2 (6) | 2 (6) |
| Grade 3+ nonhematologic, N (%) | 10 (29) | 4 (11) | 18 (51) | 10 (29) |
| Grade 4+ Nonhematologic, N (%) | 2 (6) | 2 (6) | 1 (3) | 0 (0) |
| Frequency of AEs . | Regardless of attribution . | At least possibly related . | Regardless of attribution . | At least possibly related . |
|---|---|---|---|---|
| Evaluable, N (%) | 35 (100) | 35 (100) | 35 (100) | 35 (100) |
| Grade 3+, N (%) | 15 (43) | 9 (26) | 26 (74) | 19 (54) |
| Grade 4+, N (%) | 2 (6) | 2 (6) | 3 (9) | 2 (6) |
| Grade 3+ hematologic, N (%) | 6 (17) | 5 (14) | 15 (43) | 13 (37) |
| Grade 4+ hematologic, N (%) | 0 (0) | 0 (0) | 2 (6) | 2 (6) |
| Grade 3+ nonhematologic, N (%) | 10 (29) | 4 (11) | 18 (51) | 10 (29) |
| Grade 4+ Nonhematologic, N (%) | 2 (6) | 2 (6) | 1 (3) | 0 (0) |
There were no on-study deaths.
Adverse Events. (A-B) Distribution of all grades of the most common toxicities considered at least possibly related to the drug administration; (A) Arm B, 4 mg and (B) Arm C, 5.5 mg. (C-D) Incidence of hematological toxicity across individual cycles, highlighting lack of any cumulative hematological toxicity; (C) Arm B, 4 mg and (D) Arm C, 5.5 mg.
Adverse Events. (A-B) Distribution of all grades of the most common toxicities considered at least possibly related to the drug administration; (A) Arm B, 4 mg and (B) Arm C, 5.5 mg. (C-D) Incidence of hematological toxicity across individual cycles, highlighting lack of any cumulative hematological toxicity; (C) Arm B, 4 mg and (D) Arm C, 5.5 mg.
Dosing of ixazomib during the first 12 cycles. (A) Arm B. (B) Arm C.
Dosing of ixazomib during the first 12 cycles. (A) Arm B. (B) Arm C.
Discussion
PIs, especially in combination with other myeloma drugs, both new and old, have become an integral part of myeloma therapies in the up-front setting as well as in the relapsed setting. Introduction of ixazomib now allows for use of this important class of drug in a more convenient fashion and, especially in combination with IMiDs or alkylators, enables development of highly effective, all-oral regimens. Although it is clear that triplets incorporating a PI and an IMiD are the optimal choice in many settings, there continues to be a role for these 2 classes of drugs to be used in combination with dexamethasone in patients unable to tolerate the triplet combinations because of age or comorbidities.22 Ixazomib has been studied as a single agent, given once or twice weekly, in the initial phase 1 studies; in the once-weekly trial, the maximum tolerated dose was the equivalent of the flat dose of 5.5 mg given weekly for 3 of 4 weeks.18,20 We previously examined, in a phase 2 study, the utility of adding dexamethasone to ixazomib in patients with relapsed myeloma.23 We were able to demonstrate improved efficacy when weekly dexamethasone was added to standard doses of ixazomib. Although the dose of ixazomib currently approved for use in combination with lenalidomide is 4 mg weekly, it is not clear if higher doses could be tolerated with improved efficacy when combined with dexamethasone alone in a doublet. We designed the current study to ask 2 important questions: (1) can we improve on the efficacy of ixazomib by adding dexamethasone right from the beginning instead of adding it for lack of adequate response and (2) can we examine the efficacy and toxicity of the higher dose of 5.5 mg of ixazomib in combination with dexamethasone?
The current study supports the clinical utility of the doublet of ixazomib and dexamethasone and provides relevant information that can inform the clinical use of the drug. Although results of the phase 3 trial support the use of ixazomib in combination with lenalidomide and dexamethasone, the results of the current trial do provide justification for exploring the doublet in patients with myeloma in future clinical trials to better define its use. Of importance, the results also allow us to get a good estimate of the efficacy of these 2 doses of ixazomib in the setting of relapsed myeloma, which will form a reference point as other combinations with ixazomib are being evaluated in ongoing and future trials. The results of the current trial, placed in the context of the phase 1 trial of single-agent ixazomib, and the prior phase 2 trial of single-agent ixazomib with dexamethasone added for inadequate response, provide a valuable set of information, even though they cannot be formally compared. The ORR with the on-demand approach of adding dexamethasone was 34%, with a CBR of 47% including the 4 patients with an MR. In the current study, we saw a similar rate of efficacy using the combination of 4 mg ixazomib and weekly dexamethasone, with 31% ORR and a 46% CBR including MR. Of interest, the response rate appears to be higher among those receiving the 5.5 mg dose of ixazomib, with a 54% ORR and a 57% CBR. Similarly, the proportion of patients with deeper responses (VGPR or better) was also higher in patients receiving a high dose: 23% for low-dose arm and 31% for the high-dose arm.
It is also important to place the current results in perspective with what has been observed with the doublets in this population. The ORR with bortezomib and dexamethasone was 63% in the Phase 3 Study With Carfilzomib and Dexamethasone Versus Bortezomib and Dexamethasone for Relapsed Multiple Myeloma Patients (ENDEAVOR) trial, but this was a relatively less treated group of patients with 103 prior treatment compared with the median of 4 prior therapies in the current trial.24 The PFS was 9.4 months in the ENDEAVOR trial compared with approximately 6 months in the current phase 3 trial. In contrast, the combination of carfilzomib used at a higher dose in ENDEAVOR resulted in a PFS of 18.7 months and response rate of 77%. In contrast, in the Assessment of Proteasome Inhibition for Extending Remissions study, the ORR was 38% with a time to progression of 6.2 months and may be more comparable with the results seen here.6 Although it is difficult to compare the patient populations in the different trials, lenalidomide and dexamethasone in the MM09 trial resulted in a 61% ORR.25 In contrast, pomalidomide and low-dose dexamethasone in a patient population more comparable to the population studied here led to an ORR of 31% and a PFS of 4 months.26
The differences in the response rate need to be balanced with the toxicity profile of the study arms. The main toxicities that we observed here are in line with the previous experience with this drug, including fatigue, thrombocytopenia, nausea, and diarrhea.18,20 Peripheral neuropathy considered at least possibly related to ixazomib was mostly limited to grades 1 and 2, although we saw 2 grade 3 events in the high-dose arm. There was no cumulative hematological toxicity and the thrombocytopenia usually recovered before the next cycle. The overall incidence of AEs, particularly hematological- and gastrointestinal-related toxicity, appears to be higher with the 5.5-mg dose compared with a 4-mg dose. In each dose level, there were 2 grade 4 AEs considered to be related to the drug. Both in the 4-mg arm were nonhematological, whereas both in the 5.5-mg arm were hematological. Overall, dose reductions of ixazomib were more commonly seen in the higher dose arm. Nearly 40% of the patients in the high-dose arm had their ixazomib dose reduced to 4 mg by the end of cycle 4. In terms of the drug delivery, 95% of the intended cumulative dose of ixazomib was delivered in the 4-mg arm compared with 85% in the 5.5 mg arm.
In conclusion, ixazomib in combination with dexamethasone has promising activity in relapsed myeloma, along with a favorable toxicity profile. Given the convenience of an oral route and once-weekly dosing, this regimen can play a role in the management of older patients and frailer patients as well as in patients with more indolent relapses. A higher dose of ixazomib at 5.5 mg along with weekly dexamethasone of 40 mg appears to have a higher response rate when used as a doublet in relapsed MM, albeit with higher toxicity. Careful attention needs to be given to the toxicity associated with the higher dose, and timely dose reductions will be required to mitigate the adverse effects. Future studies should compare this doublet to other doublets containing a novel agent currently in use in this patient population.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by Takeda Pharmaceuticals and Mayo Clinic Hematological Malignancies Program, National Cancer Institute, National Institutes of Health (grants CA107476 and CA168762).
Authorship
Contribution: S.K.K. designed the research, was the principal investigator for the trial, and wrote the manuscript. B.R.L. and A.E.H. were involved in the design and analysis of the trial and contributed to the manuscript writing. V.R., C.B.R., M.Q.L., M.A.G., M.A.T., T.E.W., F.B., C.E.R., J.M., P.L.B., P.K., J.C., K.S., S.R.H., Y.L.H., W.G., S.A., D.D., R.S.G., Y.L., S.V.R., and A.D. were involved with the study conduct, enrolled patients, reviewed the analysis, and in the manuscript preparation.
Conflict-of-interest disclosure: S.K.K. has research funding from Celgene, Takeda, Novartis, Merck, Sanofi, and Janssen (to institution) and has participated in advisory boards with no personal compensation (Celgene, Takeda, Sanofi, Glycomimetics, and Janssen). P.L.B. has consulted for BMS and Janssen. J.M. has grant funding from Celgene, Abbvie, Sanofi, and Onyx. The remaining authors declare no competing financial interests.
Correspondence: Shaji Kumar, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55906; e-mail: kumar.shaji@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal