Key Points
MOZ deletion in adult mice leads to a rapid loss of cells with HSC cell surface immuno-phenotype and transplantation ability.
Absence of classically defined HSCs for an extended period does not substantially affect steady-state hematopoiesis.
Abstract
Hematopoietic stem cells (HSCs) are conventionally thought to be at the apex of a hierarchy that produces all mature cells of the blood. The quintessential property of these cells is their ability to reconstitute the entire hematopoietic system of hemoablated recipients. This characteristic has enabled HSCs to be used to replenish the hematopoietic system of patients after chemotherapy or radiotherapy. Here, we use deletion of the monocytic leukemia zinc finger gene (Moz/Kat6a/Myst3) to examine the effects of removing HSCs. Loss of MOZ in adult mice leads to the rapid loss of HSCs as defined by transplantation. This is accompanied by a reduction of the LSK-CD48–CD150+ and LSK-CD34–Flt3– populations in the bone marrow and a reduction in quiescent cells in G0. Surprisingly, the loss of classically defined HSCs did not affect mouse viability, and there was no recovery of the LSK-CD48–CD150+ and LSK-CD34–Flt3– populations 15 to 18 months after Moz deletion. Clonal analysis of myeloid progenitors, which produce short-lived granulocytes, demonstrate that these are derived from cells that had undergone recombination at the Moz locus up to 2 years earlier, suggesting that early progenitors have acquired extended self-renewal. Our results establish that there are essential differences in HSC requirement for steady-state blood cell production compared with the artificial situation of reconstitution after transplantation into a hemoablated host. A better understanding of steady-state hematopoiesis may facilitate the development of novel therapies engaging hematopoietic cell populations with previously unrecognized traits, as well as characterizing potential vulnerability to oncogenic transformation.
Introduction
Because the majority of mature blood cells have short life spans, very large numbers of cells are produced throughout life.1 Conventionally, hematopoiesis has been thought to be supported by rare stem cells, which seldom enter the cell cycle,2 but are at the apex of a hierarchy that produces rapidly cycling progenitors.3 Primitive progenitors are thought to differentiate into progressively more lineage-restricted progenitors, which proliferate rapidly but lack long-term self-renewal capability. This model of hematopoiesis has been developed through studies of bone marrow transplantation into lethally irradiated recipients. A single hematopoietic stem cell (HSC), prospectively identified and purified on the basis of cell surface markers, can reconstitute the entire hematopoietic system of a recipient.4 However, recent studies that have avoided the use of transplantation assays suggest that steady-state hematopoiesis in healthy animals may be supported primarily by lineage-committed progenitors that have unexpectedly long lifetimes.5-7 The requirement for transplantable HSCs during adulthood has not been formally tested, as the phenotype of adult mice without HSCs has not been reported. Furthermore, clonal analysis of HSCs has revealed an underappreciated level of heterogeneity within the stem cell compartment, including lineage-biased stem cells.8-11 Thus, the physiologic requirement of HSCs in healthy animals and the relationship between “classically” defined transplantable HSCs and early progenitors is unclear.
The monocytic leukemia zinc finger gene (MOZ/KAT6A/MYST3) was first identified in a recurrent chromosomal translocation (t8;16)(p11;p13) associated with acute myeloid leukemia, where it is fused to the CREB binding protein.12 This subtype of acute myeloid leukemia has a very poor prognosis.13,14 In common with many genes that lead to leukemia when mutated, Moz has an essential function in hematopoiesis. Mice homozygous for Moz loss of function mutations fail to develop HSCs during embryogenesis.15-17 Surprisingly, hematopoietic progenitors develop in the absence of MOZ early in development, albeit in reduced numbers, and these are able to form all mature blood cell types. In particular, Moz null fetuses have a normal hematocrit, although there is a delay in erythrocyte maturation. These results show that MOZ is indispensable, specifically, for the development of HSCs, but is less important for the differentiation of progenitors.15
MOZ is a lysine acetyltransferase required for histone 3, lysine 9 acetylation at target loci,18,19 in particular at Hox loci.20 MOZ is a global activator of Hox gene expression and the absence of MOZ results in homeotic transformation of body segment identity.20 The function of MOZ resembles that of the chromatin activator and trithorax group protein MLL1 (KTM2A). Indeed, like MOZ, MLL1 activates Hox gene expression and specifies body segment identity.21 MLL1 is the target of recurrent translocations causing aggressive forms of leukemia22 and is essential for maintenance of HSCs.23-25 MOZ cooperates with MLL1 to regulate gene expression both during embryogenesis and in the hematopoietic system.20,26,27 Conversely, a chromatin repressor opposing the action of trithorax group proteins, the polycomb group protein BMI1 (PCGF4), also regulates body segment identity and is required for the maintenance of HSC function.28-30 BMI1 directly opposes the function of MOZ in Hox gene regulation during embryonic development,31 although, interestingly, both BMI1 and MOZ are suppressors of cellular senescence.32-34 Thus, MOZ appears to be an essential player in the network of trithorax and polycomb chromatin modifying proteins that regulate HSCs and maintain hematopoietic homeostasis.
In this study, we use the loss of MOZ to examine the effect of specifically removing classically defined, transplantable HSCs on steady state hematopoiesis. We define HSCs as cells that are both capable of reconstituting both lymphoid and myeloid lineages after transplantation into lethally irradiated recipient mice and able to functionally compete with other HSCs. Our experiments established that deletion of Moz leads to a rapid loss of adult bone marrow HSCs, defined both by characteristic cell surface markers and also functionally through transplantation assays; however, lineage-committed progenitors are largely unaffected. We used the unique situation of the absence of HSCs in Moz deleted mice to examine the role that adult HSCs might play in steady-state hematopoiesis. We show that the absence of classically defined HSCs has a relatively minor effect on steady-state hematopoiesis.
Methods
Mice
Experiments were approved by the Walter and Eliza Hall Animal Ethics Committee and conformed to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Mice were on the C57BL/6 genetic background and kept in a 14-hour light and 10-hour dark cycle at 22°C. To induce cre-recombinase expression, mice were given 3 intraperitoneal injections of 2.5 mg/kg body weight poly(I:C) (GE Healthcare) in 0.9% saline separated by 48 hours. Using this protocol, mice did not lose >10% of their initial body weight. Polymerase chain reaction (PCR) genotyping was as follows: wild-type allele Moz+, 348 bp; loxP-flanked Mozfl allele, 447 bp; null allele recombined in the Mozfl;Mx1-cre hematopoietic system Moz–, 172 bp. All 3 products can be detected in a single PCR using forward primer MozA, TTCTTGACCTCTGTGTCGTGTGC, and reverse primers MozB, AGAAGTACAGTGCTTTGGTTTCC, and MozC, ATAGGAACTTCATCAGTCAGGTAC. Recombination efficiency was determined by quantitative PCR (qPCR) to determine levels of the intact allele, using the SYBR green protocol (Bioline). In all experiments, the extent of recombination in blood and bone marrow of donor mice and after transplantation in recipient mice was assessed. In 3 instances, recombination was poor, and these animals were removed from subsequent analysis.
Transplantation assays
For competitive transplantation assays, 105 or 5 × 105 wild-type cells marked by CD45.1, as indicated, were mixed with 2 × 106 cells from CD45.2 Mozfl/fl;Mx1-cre, or Mozfl/fl or Moz+/+;Mx1-cre mice and intravenously injected into lethally irradiated (2 doses: 5.5 Gy separated by 3 hours) CD45.1 or CD45.1/2 C57BL/6 congenic recipients. Moz+/+;Mx1-cre control cells competitively (1:1) transplanted after treatment using the same poly(I:C) protocol did not show any differences in repopulating capacity (supplemental Figure 1, available on the Blood Web site). Moribund recipient mice suffering from >15% weight loss and anemia were killed. Flow cytometry was performed as described in the supplemental Data.
Colony assays
Colony cell-forming assays were performed at 72 hours (Figure 2) or 4 months after the final poly(I:C) injection (supplemental Figure 3) or 4.3 months after transplantation of bone marrow that had been treated with poly(I:C) in the recipient 18 months prior to transplantation (Figure 5; supplemental Figure 10). A total of 20 000 bone marrow cells were cultured in 0.3% agar and stimulated by the addition of purified murine growth factors, interleukin 3 (IL3; 10−3 U/mL), stem cell factor (SCF; 100 ng/mL), and erythropoietin (EPO; 2 U/mL). Cultures were incubated for 7 days, and then fixed, stained, and counted as described previously.35
Statistical analyses
Statistical analyses used Stata v12 (Stata Corporation). Data were analyzed using 1-way factorial analysis of variance, with Moz genotype as the independent factor, followed by Bonferroni’s post hoc test or by regression analysis, in time course experiments.
Results
Loss of Moz leads to rapid loss of transplantable stem cells
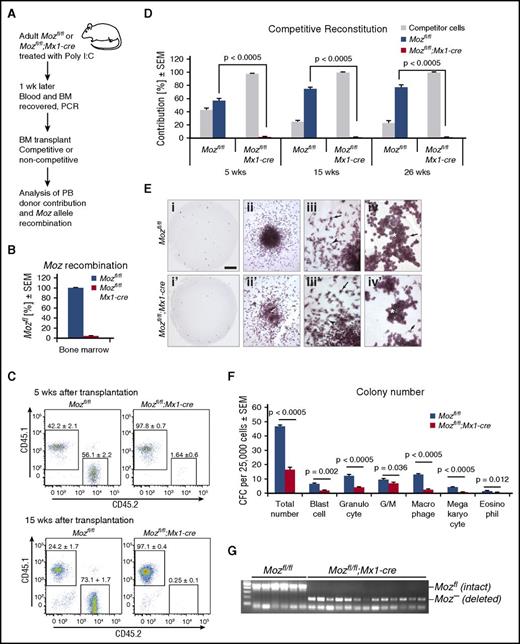
We excised Moz exons 3 to 7, which were flanked by loxP sites in the Mozfl allele20 using the interferon-responsive Mx1-cre transgene.36 Inducing deletion with poly(I:C) in the hematopoietic system (Figure 1A) resulted in efficient deletion of Moz in bone marrow (Figure 1B). Mozfl/fl;Mx1-cre-negative and Mozfl/fl;Mx1-cre-positive (termed Mozfl/fl and Mozfl/fl;Mx1-cre, respectively) donors were treated 3 times (48 hours apart) with poly(I:C), and their bone marrow was transplanted 72 hours after the last injection into lethally irradiated recipients. Recipient, competitor, and test donor cells were distinguished by their cell surface CD45.1 and CD45.2 phenotype. In competitive transplants, 2 × 106 test cells together with 5 × 105 competitor cells were injected. Recipient mice were analyzed at 5, 15, and 26 weeks after transplantation. As expected, Moz-intact Mozfl/fl donor cells competed effectively and after 26 weeks contributed 77% of peripheral blood cells (Figure 1D). In contrast, no Mozfl/fl;Mx1-cre donor cells were detected 15 and 26 weeks after transplantation (<0.3%; Figure 1C-D). In addition, Mozfl/fl;Mx1-cre donor cells were unable to reconstitute the hematopoietic system of lethally irradiated recipients in the absence of competitor (supplemental Figure 1). Induction of Cre recombinase in Mx1-cre transgenic mice that were wild type at the Moz locus had no effect on hematopoietic repopulating activity (supplemental Figure 1).
Acute deletion of Moz results in the complete absence of transplantable HSCs. (A) Experimental design. Bone marrow from donor Mozfl/fl;Mx1-cre (n = 6) and control Mozfl/fl (n = 6) mice were used. Each donor bone marrow sample was transplanted into 3 recipients. (B) Genomic Moz deletion efficiency was 95.8 ± 3.2% in animals used in A assessed by qPCR; the residual 4.2% unrecombined alleles were derived from nonhematopoietic and interferon unresponsive cells. (C) Flow cytometric analysis of peripheral blood of recipient mice 5 and 15 weeks after competitive transplantation (1:4 ratio of competitor to test cells). (D) Quantitation of donor contribution to the peripheral blood of lethally irradiated mice at 5, 15, and 26 weeks after competitive transplantation. Note the absence of contribution from Mozfl/fl;Mx1-cre bone marrow cells after 15 and 26 weeks. (E) Moz-deleted bone marrow contains myeloid colony-forming cells, albeit at reduced numbers. Mozfl/fl (i-iv) and Mozfl/fl;Mx1-cre cultures (i′-iv′) are shown after stimulation with SCF, EPO, and IL3. Comparison of typical plates. Note the reduction in colony number in the Mozfl/fl;Mx1-cre cultures (i vs i′). Cultures of both genotypes contain cells with high proliferative capacity (ii vs ii′). Overview of a colony (iii vs iii′). Morphology of macrophages (arrow) and granulocytes (arrowhead) differentiating from cells of either genotype was similar (iv vs iv′). Likewise, morphology of cells (arrows) within blast cell colonies (asterisk) was indistinguishable between genotypes. (F) Enumeration of colonies in Mozfl/fl and Mozfl/fl;Mx1-cre cultures. (G) Genotyping of colonies, showing efficient recombination of the Mozfl/fl allele after poly(I:C) induction of Mx1-driven cre-recombinase. Data are presented as the mean ± standard error of the mean (SEM).
Acute deletion of Moz results in the complete absence of transplantable HSCs. (A) Experimental design. Bone marrow from donor Mozfl/fl;Mx1-cre (n = 6) and control Mozfl/fl (n = 6) mice were used. Each donor bone marrow sample was transplanted into 3 recipients. (B) Genomic Moz deletion efficiency was 95.8 ± 3.2% in animals used in A assessed by qPCR; the residual 4.2% unrecombined alleles were derived from nonhematopoietic and interferon unresponsive cells. (C) Flow cytometric analysis of peripheral blood of recipient mice 5 and 15 weeks after competitive transplantation (1:4 ratio of competitor to test cells). (D) Quantitation of donor contribution to the peripheral blood of lethally irradiated mice at 5, 15, and 26 weeks after competitive transplantation. Note the absence of contribution from Mozfl/fl;Mx1-cre bone marrow cells after 15 and 26 weeks. (E) Moz-deleted bone marrow contains myeloid colony-forming cells, albeit at reduced numbers. Mozfl/fl (i-iv) and Mozfl/fl;Mx1-cre cultures (i′-iv′) are shown after stimulation with SCF, EPO, and IL3. Comparison of typical plates. Note the reduction in colony number in the Mozfl/fl;Mx1-cre cultures (i vs i′). Cultures of both genotypes contain cells with high proliferative capacity (ii vs ii′). Overview of a colony (iii vs iii′). Morphology of macrophages (arrow) and granulocytes (arrowhead) differentiating from cells of either genotype was similar (iv vs iv′). Likewise, morphology of cells (arrows) within blast cell colonies (asterisk) was indistinguishable between genotypes. (F) Enumeration of colonies in Mozfl/fl and Mozfl/fl;Mx1-cre cultures. (G) Genotyping of colonies, showing efficient recombination of the Mozfl/fl allele after poly(I:C) induction of Mx1-driven cre-recombinase. Data are presented as the mean ± standard error of the mean (SEM).
To determine whether, like mice bearing a hypomorphic allele of cKit,37,38 Mozfl/fl;Mx1-cre mice were able to act as transplant recipients without irradiation, we transplanted 2 × 106 CD45.1 bone marrow cells into Mozfl/fl;Mx1-cre and control mice 72 hours after the last poly(I:C) injection. Three months after transplantation, there was no significant CD45.1 contribution to peripheral blood: 0.02 ± 0.004% Mozfl/fl controls vs 0.38 ± 0.33% Mozfl/fl;Mx1-cre recipients. Bone marrow from these recipient mice was transplanted into lethally irradiated CD45.1/CD45.2 recipients. All 6 recipients receiving bone marrow from Mozfl/fl;Mx1-cre mice became moribund between 19 and 28 days after transplantation, whereas 6 animals receiving Mozfl/fl control bone marrow survived. No contribution from the original CD45.1 donors was detected, showing that 3 months after poly(I:C) treatment, Mozfl/fl;Mx1-cre bone marrow mice did not harbor HSCs from the wild-type donor.
We examined the ability of both Lin–cKIT+CD48–CD150+ and Lin–cKIT+CD48+CD150+ cells from the central bone marrow and the endosteal niches to home 72 hours after induction of Moz recombination by poly(I:C) injection, as described previously.39 Although the number of Lin–cKIT+CD48–CD150+ cells within the Mozfl/fl;Mx1-cre donors was reduced compared with controls, we observed no significant difference (P > .05) in the ability of Mozfl/fl;Mx1-cre donor cells and Mozfl/fl control cells to migrate to the bone marrow (supplemental Figure 2). In addition, examination of the kinetics of Moz-deleted thymocytes suggested lymphoid progenitors were able to circulate normally between the bone marrow and the thymus (supplemental Figure 2).
Acute loss of MOZ reduces the number of colony-forming cells but not their ability to differentiate
To examine the role of MOZ in hematopoietic progenitors without the requirement for transplantation, we induced recombination of the Mozfl allele and seeded bone marrow in colony-forming assays.35 Mozfl/fl;Mx1-cre bone marrow contained substantially fewer colony-forming cells (Figure 1 E-F). However, both Mozfl/fl;Mx1-cre and Mozfl/fl bone marrow produced the full range of colony types and sizes, including large colonies, which represent progenitors with high proliferative capacity. The morphology of differentiated myeloid cell types was indistinguishable between genotypes (Figure 1E). To ensure that colonies were derived from Moz-deleted progenitors, a selection of small, medium and large colonies were picked from Mozfl/fl;Mx1-cre cultures and Mozfl/fl cultures. In each case, the colony contained only Moz-deleted cells when derived from the Mozfl/fl:Mx1-cre genotype (Figure 1G). This showed that, although Moz deletion affected progenitor cell number, it did not eradicate progenitor cells and did not affect lineage specification.
Loss of MOZ leads to a loss of cells in G0 phase of the cell cycle and a reduction in cells with cell surface markers characteristic of HSCs
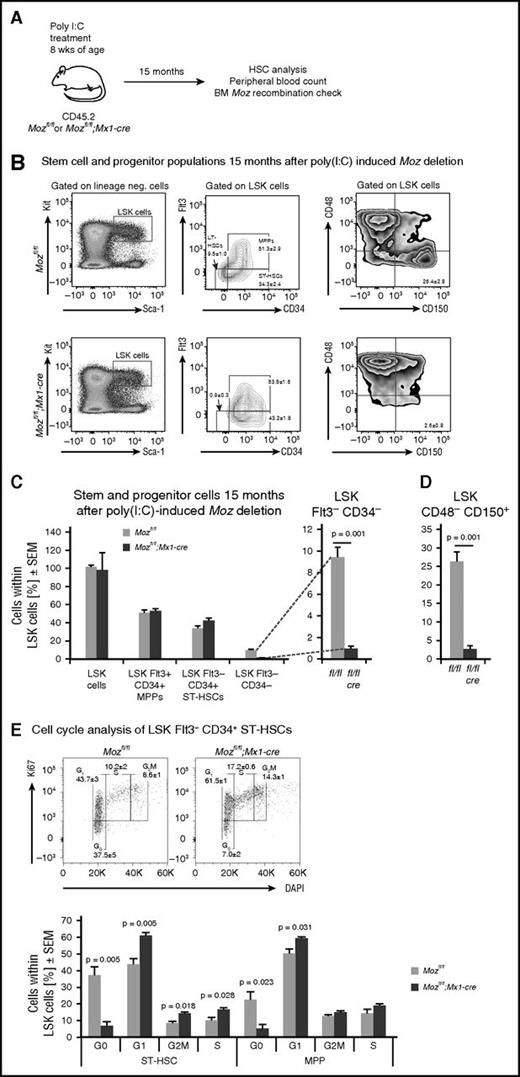
Long-term repopulating HSCs reside in bone marrow populations defined by cell surface markers. HSCs are enriched in the lineage-negative, cKIT-positive, SCA1-positive, CD48-negative, CD150-positive (LSK-CD48–CD150+) cell population, and up to half the LSK-CD48–CD150+ cells are HSCs.40,41 HSCs are also enriched in the LSK-CD34–FLT3– population.42,43 HSCs are responsive to interferon stimulation.44,45 Therefore, we waited 4 months for the effects of poly(I:C) injection to abate before performing a detailed examination of the HSC compartment (Figure 2A). To examine whether the absence of MOZ affected hematopoietic progenitors after 4 months, we performed colony-forming assays. We observed no difference in the number and size of myeloid colonies (supplemental Figure 3) between genotypes. Large colonies, representing highly proliferative colony-forming cells, were picked and genotyped. The loxP sites in the Moz locus had efficiently recombined in colonies derived from Mozfl/fl;Mx1-cre mice treated with poly(I:C) (Figure 2B). One colony out of a total of 18 picked from Mozfl/fl;Mx1-cre bone marrow was heterozygous; no colonies contained completely unrecombined alleles.
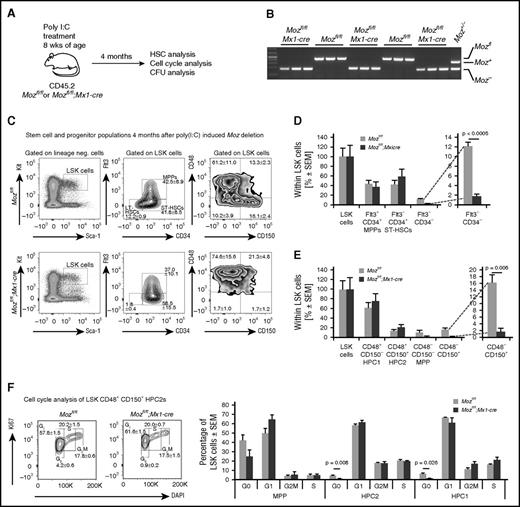
Deletion of Moz results in a substantial reduction in populations with a cell surface phenotype characteristic of HSCs and of cells in G0. (A) Experimental design. Mice were treated with poly(I:C) and then left undisturbed for 4 months to allow recovery from acute inflammation induced by poly(I:C). (B) Examples of PCR genotyping of progenitor colonies from Mozfl/fl and Mozfl/fl;Mx1-cre cultures after stimulation with SCF, EPO, and IL3. Note that myeloid colony-forming progenitors (supplemental Figure 3) are derived from Moz-deleted cells that have existed for 4 months. (C) Flow cytometry plots showing analyses of the LSK-CD34–FLT3– and LSK-CD48–CD150+ populations in Mozfl/fl and Mozfl/fl;Mx1-cre bone marrow cells. (D-E) Quantification of stem cell surface markers by flow cytometry of recipients of Mozfl/fl and Mozfl/fl;Mx1-cre cells. Note the absence of typical LSK-CD34–FLT3– and LSK-CD48–CD150+ populations. (F) Cell cycle analysis of stem/progenitor populations. Note the reduction in the number of cells in G0. Data are presented as the mean ± SEM.
Deletion of Moz results in a substantial reduction in populations with a cell surface phenotype characteristic of HSCs and of cells in G0. (A) Experimental design. Mice were treated with poly(I:C) and then left undisturbed for 4 months to allow recovery from acute inflammation induced by poly(I:C). (B) Examples of PCR genotyping of progenitor colonies from Mozfl/fl and Mozfl/fl;Mx1-cre cultures after stimulation with SCF, EPO, and IL3. Note that myeloid colony-forming progenitors (supplemental Figure 3) are derived from Moz-deleted cells that have existed for 4 months. (C) Flow cytometry plots showing analyses of the LSK-CD34–FLT3– and LSK-CD48–CD150+ populations in Mozfl/fl and Mozfl/fl;Mx1-cre bone marrow cells. (D-E) Quantification of stem cell surface markers by flow cytometry of recipients of Mozfl/fl and Mozfl/fl;Mx1-cre cells. Note the absence of typical LSK-CD34–FLT3– and LSK-CD48–CD150+ populations. (F) Cell cycle analysis of stem/progenitor populations. Note the reduction in the number of cells in G0. Data are presented as the mean ± SEM.
Flow cytometric analysis showed that the Mozfl/fl;Mx1-cre bone marrow showed a marked reduction in LSK-CD34–FLT3– (6.8-fold reduction) and LSK-CD48–CD150+ (9.5-fold reduction) compared with controls (Figure 2C-E). HSCs are characteristically quiescent, dividing only approximately every 145 days,2 and so a high proportion of LSK cells reside in G0. To determine the effect of loss of MOZ on cell cycle parameters, we assessed the proportion of LSK-CD48–CD150+ stem cells and progenitor subsets in various stages of the cell cycle. In each subset defined by signalling lymphocyte activation molecule markers, we found a significant reduction in the proportion of cells in G0 in the Mozfl/fl;Mx1-cre bone marrow (Figure 2F). These results show that the reduction in phenotypic HSCs, defined by cell surface markers, had no long-term effect on the number of lineage specific progenitors and that MOZ has a role in cell cycle regulation.
Absence of MOZ function does not lead to bone marrow failure
Because deletion of Moz led to the absence of classically defined HSCs, we expected that Mx1-cre deletion of Moz in situ might lead to stem cell exhaustion and anemia. To examine the effects of removing HSCs, we induced recombination of Moz in a cohort of mice and observed them over a period of 1.5 years (558 days; Figure 3A-B). Unexpectedly, Mozfl/fl;Mx1-cre mice did not become anemic. Examination of peripheral blood parameters showed hematocrit and neutrophil counts were normal, although leukocyte counts were reduced by approximately half, due to a reduction in lymphocytes (Figure 3C). Eosinophilic, basophilic granulocyte, and platelet counts were reduced. In all other respects, blood parameters were normal (supplemental Figure 4). The proportion of peripheral blood leukocytes containing undeleted Mozfl alleles remained the same between 100 and 558 days after induction of recombination, showing that there was no evidence of a selective advantage of any remaining HSCs containing intact Mozfl alleles (Figure 3B; supplemental Figure 5). Note that the peripheral blood of Mozfl/fl;Mx1-cre mice treated with poly(I:C) still contained undeleted Mozfl alleles in lymphoid cells. Comparison of the extent of Moz deletion in the bone marrow with the extent of Moz deletion in peripheral blood lymphocytes and granulocytes suggests these circulating lymphocytes were generated prior to initiation of recombination (supplemental Figure 6).
Loss of HSCs has little effect during the normal lifespan of mice. (A) Experimental design of Moz deletion in situ. (B) Quantification of Mozfl alleles in nucleated cells of the peripheral blood by qPCR from 100 to 558 days after poly(I:C) treatment of Mozfl/fl;Mx1-cre (n = 5) and Mozfl/fl (n = 6) control mice. No significant change in the level of Moz-deleted alleles was seen over this period (P = .9775). (C) Peripheral blood analysis of mice 18 months after poly(I:C) treatment of (n = 9) Mozfl/fl;Mx1-cre and (n = 9) Mozfl/fl control mice (complete analysis; supplemental Figure 4). The erythrocyte (RBC) and neutrophil counts were normal. Note the reduction in leukocytes (white blood cells [WBCs]), due to a reduction in lymphocytes (predominantly B cells; supplemental Figure 6). (D) Experimental design of chimeric transplantation first and then Moz deletion 90 days later. The bone marrow from each donor Mozfl/fl;Mx1-cre (n = 3) and control Mozfl/fl (n = 3) mouse was transplanted into 3 recipients together with wild-type CD45.1 bone marrow, as indicated. (E) Percentage of CD45.2-positive Mozfl/fl;Mx1-cre, and Mozfl/fl control cells in peripheral blood changes over time in the presence of wild-type competitors. Data are presented as the mean ± SEM.
Loss of HSCs has little effect during the normal lifespan of mice. (A) Experimental design of Moz deletion in situ. (B) Quantification of Mozfl alleles in nucleated cells of the peripheral blood by qPCR from 100 to 558 days after poly(I:C) treatment of Mozfl/fl;Mx1-cre (n = 5) and Mozfl/fl (n = 6) control mice. No significant change in the level of Moz-deleted alleles was seen over this period (P = .9775). (C) Peripheral blood analysis of mice 18 months after poly(I:C) treatment of (n = 9) Mozfl/fl;Mx1-cre and (n = 9) Mozfl/fl control mice (complete analysis; supplemental Figure 4). The erythrocyte (RBC) and neutrophil counts were normal. Note the reduction in leukocytes (white blood cells [WBCs]), due to a reduction in lymphocytes (predominantly B cells; supplemental Figure 6). (D) Experimental design of chimeric transplantation first and then Moz deletion 90 days later. The bone marrow from each donor Mozfl/fl;Mx1-cre (n = 3) and control Mozfl/fl (n = 3) mouse was transplanted into 3 recipients together with wild-type CD45.1 bone marrow, as indicated. (E) Percentage of CD45.2-positive Mozfl/fl;Mx1-cre, and Mozfl/fl control cells in peripheral blood changes over time in the presence of wild-type competitors. Data are presented as the mean ± SEM.
To examine the effect of deleting Moz in the presence of wild-type competitor cells, we generated bone marrow chimeras, in which the wild-type competitor cells were CD45.1 positive and the Mozfl/fl;Mx1-cre test or Mozfl/fl control cells were CD45.2 positive. We allowed these mice to recover for 90 days. We then induced recombination of the Mozfl locus by injection of poly(I:C) and assayed the peripheral blood at 30, 60, 120, 180, and 250 days after poly(I:C) injection (Figure 3D). In the recipients that received Mozfl/fl;Mx1-cre bone marrow, there was a steady and significant decline (P < .0001) in the proportion of CD45.2 cells in the blood (Figure 3E). In contrast, the Mozfl/fl control bone marrow contribution showed a tendency to increase over time (Figure 3E), as expected of CD45.2 bone marrow compared with CD45.1 bone marrow, based on the literature.46 The results show that the MOZ deleted cells are compromised in the production of mature blood leukocytes.
Mice lacking MOZ for an extended period do not regain classically defined HSCs
To examine whether the Moz-deleted hematopoietic cells, which were unable to reconstitute a lethally irradiated host 72 hour or 3 months after Moz deletion, but could sustain hematopoiesis for ≥18 months in situ, had regained full HSC potential, we examined cell surface markers of stem cell populations in the bone marrow and tested the bone marrow functionally in competitive and noncompetitive transplantation experiments between 15 and 18 months after Moz deletion.
Flow cytometric analysis showed that 15 months after induction of recombination, the Mozfl/fl;Mx1-cre bone marrow showed continued suppression of cells with HSC cell surface markers, namely LSK-CD34–FLT3– (10.6-fold reduction) and LSK-CD48–CD150+ (10.2-fold reduction) compared with controls (Figure 4A-D). Note that although in wild-type mice the reconstitution activity declines with age, the LSK-CD48–CD150+ cell surface markers still define HSCs.47 As was the case at 4 months after recombination, cell cycle analysis showed that the remaining population of Moz-deleted cells in the stem/progenitor compartment lacked cells in G0 (Figure 4E).
The number of cells with HSC cell surface markers is not restored after 15 months without MOZ. (A) Experimental design. Mice were treated with poly(I:C) and then left undisturbed for 15 months; bone marrow stem cell populations and stage of the cell cycle were then quantitated in 3 Mozfl/fl;Mx1-cre and 3 control Mozfl/fl mice. (B) Flow cytometry plots showing analyses of the LSK-CD34–FLT3– and the LSK-CD48–CD150+ populations in Mozfl/fl and Mozfl/fl;Mx1-cre bone marrow 15 months after poly(I:C) treatment. Note that these are similar to those shown in Figure 2. (C-D) Quantification of stem cell markers by flow cytometry of Mozfl/fl and Mozfl/fl;Mx1-cre bone marrow cells. Note the reduction in typical LSK-CD34–FLT3– and LSK-CD48–CD150+ populations. (E) Cell cycle analysis of stem/progenitor populations. Note the reduction in the number of cells in G0. Data are presented as the mean ± SEM.
The number of cells with HSC cell surface markers is not restored after 15 months without MOZ. (A) Experimental design. Mice were treated with poly(I:C) and then left undisturbed for 15 months; bone marrow stem cell populations and stage of the cell cycle were then quantitated in 3 Mozfl/fl;Mx1-cre and 3 control Mozfl/fl mice. (B) Flow cytometry plots showing analyses of the LSK-CD34–FLT3– and the LSK-CD48–CD150+ populations in Mozfl/fl and Mozfl/fl;Mx1-cre bone marrow 15 months after poly(I:C) treatment. Note that these are similar to those shown in Figure 2. (C-D) Quantification of stem cell markers by flow cytometry of Mozfl/fl and Mozfl/fl;Mx1-cre bone marrow cells. Note the reduction in typical LSK-CD34–FLT3– and LSK-CD48–CD150+ populations. (E) Cell cycle analysis of stem/progenitor populations. Note the reduction in the number of cells in G0. Data are presented as the mean ± SEM.
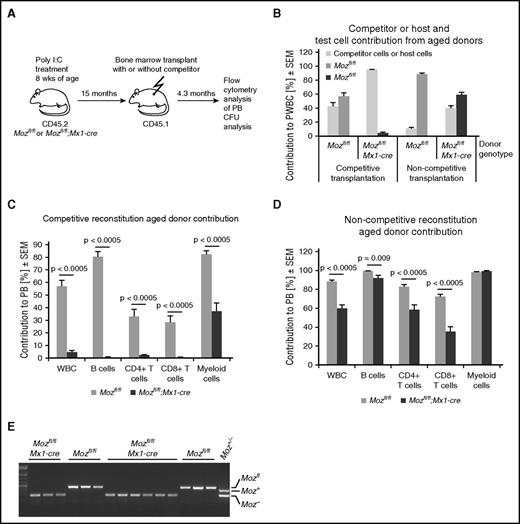
In competitive transplantations, 2 × 106 bone marrow cells from these 15-month Moz-deleted mice were combined with 5 × 105 CD45.1 competitor bone marrow cells from 8-week-old mice. In contrast to transplantation within 1 week after Moz deletion, there was a small but significant contribution (5%) from the Moz-deleted bone marrow 15 months after induction of deletion, as assessed 4 months after transplantation (Figure 5A-C). This ranged from 1% to 7% between recipients. Similar results were obtained from competitive transplantations using bone marrow from mice 18 months after Moz deletion (transplant series 2; supplemental Figure 7). A larger contribution of Mozfl/fl;Mx1-cre cells was seen when a lower number of competitor cells was used (transplant series 3; supplemental Figure 8). The contribution to CD4 T cells (2.5%) was very low, as was the contribution to CD8 T cells (0.4%) and B cells (0.7%), but there was a substantial contribution to the myeloid cell compartment (37.5%) (Figure 5C). As expected, control cells from Moz-intact bone marrow 15 (Figure 5C) and 18 months after treatment with poly(I:C) showed efficient multilineage reconstitution (supplemental Figures 7 and 8).
Aged Moz-deleted Mozfl/fl;Mx1-cre bone marrow cells acquire long-term reconstitution capability. (A) Experimental design. Mice were treated with poly(I:C) and then left undisturbed for 15 months prior to transplantation. Peripheral blood was analyzed at 4.3 months after transplantation. (B) Contribution to peripheral blood leukocytes 4.3 months after transplantation, both in the presence of 8-week-old wild-type competitor bone marrow and without competitor (noncompetitive). Note that aged Mozfl/fl control HSCs still have efficient multilineage reconstituting capacity. (C-D) Comparison of contribution of Moz-intact Mozfl/fl or Moz-deleted Mozfl/fl;Mx1-cre transplant to circulating B cells, T cells, myeloid cells, and total white blood cells (WBCs) after transplantation with (C) competitor and (D) without competitor presented as a percentage of CD45.2 (donor) contribution to peripheral blood. (C) Note that Moz-deleted bone marrow contains myeloid repopulating activity, but almost completely lacks lymphoid repopulating activity in competitive transplants. (D) However, the same cell preparations produce extensive multi-lineage contribution in the absence of exogenous competitor. (E) PCR genotyping of myeloid colonies from cultures sorted from the bone marrow of recipients analyzed in D. (E) Genotyping of Moz-intact Mozfl/fl or Moz-deleted Mozfl/fl;Mx1-cre progenitor colonies grown from cells isolated from transplant recipients. CD45.2 (donor) cells were isolated by FACS from primary recipients and cultured in the presence of SCF, EPO, and IL3 (supplemental Figure 10). Examples showing genotyping of large colonies derived from highly proliferative progenitors originally derived from control Mozfl/fl or Mozfl/fl;Mx1-cre mice treated with poly(I:C) almost 2 years earlier. Note that colonies from Mozfl/fl;Mx1-cre contain Moz deleted alleles (Figure 1G). Data are presented as the mean ± SEM.
Aged Moz-deleted Mozfl/fl;Mx1-cre bone marrow cells acquire long-term reconstitution capability. (A) Experimental design. Mice were treated with poly(I:C) and then left undisturbed for 15 months prior to transplantation. Peripheral blood was analyzed at 4.3 months after transplantation. (B) Contribution to peripheral blood leukocytes 4.3 months after transplantation, both in the presence of 8-week-old wild-type competitor bone marrow and without competitor (noncompetitive). Note that aged Mozfl/fl control HSCs still have efficient multilineage reconstituting capacity. (C-D) Comparison of contribution of Moz-intact Mozfl/fl or Moz-deleted Mozfl/fl;Mx1-cre transplant to circulating B cells, T cells, myeloid cells, and total white blood cells (WBCs) after transplantation with (C) competitor and (D) without competitor presented as a percentage of CD45.2 (donor) contribution to peripheral blood. (C) Note that Moz-deleted bone marrow contains myeloid repopulating activity, but almost completely lacks lymphoid repopulating activity in competitive transplants. (D) However, the same cell preparations produce extensive multi-lineage contribution in the absence of exogenous competitor. (E) PCR genotyping of myeloid colonies from cultures sorted from the bone marrow of recipients analyzed in D. (E) Genotyping of Moz-intact Mozfl/fl or Moz-deleted Mozfl/fl;Mx1-cre progenitor colonies grown from cells isolated from transplant recipients. CD45.2 (donor) cells were isolated by FACS from primary recipients and cultured in the presence of SCF, EPO, and IL3 (supplemental Figure 10). Examples showing genotyping of large colonies derived from highly proliferative progenitors originally derived from control Mozfl/fl or Mozfl/fl;Mx1-cre mice treated with poly(I:C) almost 2 years earlier. Note that colonies from Mozfl/fl;Mx1-cre contain Moz deleted alleles (Figure 1G). Data are presented as the mean ± SEM.
In parallel, we conducted noncompetitive transplantation experiments using 106 cells from the same donor bone marrow samples. After 4.5 months, a very substantial 60% of the cells were Mozfl/fl;Mx1-cre donor derived (Figure 5D). In contrast to competitive reconstitution, this included long-term contribution to both lymphoid (59% of CD4 T cells, 35% of CD8 T cells, and 92% of B cells, albeit with a substantial overall reduction in total B-cell numbers) and myeloid compartments (99%) in the peripheral blood of recipients (Figure 5D). The Mozfl/fl littermate control cells formed >95% of the recipient’s hematopoietic system, as expected. Similar results were obtained when competitive and noncompetitive transplants (using 2 × 106 bone marrow cells from 18-month Moz-deleted mice mixed with 5 × 105 CD45.1 competitor bone marrow cells from 8-week-old mice) were analyzed 3 or 6 months after transplantation (transplant series 2; supplemental Figure 7; gating strategy, supplemental Figure 9).
The comparison between these noncompetitive and competitive transplants suggested that the bone marrow isolated from mice that lacked classically defined HSCs for >1 year also lacked multilineage reconstituting stem cells. However, interestingly, these animals contained cells that could effectively reconstitute the myeloid compartment. To examine progenitors in the bone marrow of these recipients at a clonal level, we purified the CD45.2 cells from bone marrow by fluorescence-activated cell sorter (FACS) and cultured these cells in SCF, IL3, and EPO. In contrast to culture after acute Moz deletion, in the bone marrow of these recipients, there was no significant reduction in colony-forming cells (supplemental Figure 10). To determine the efficiency of recombination in clonally derived cells, we picked colonies, selecting for the largest colonies, which indicate a highly proliferative progenitor. A total of 72 colonies were genotyped from recipients of Mozfl/fl;Mx1-cre and Mozfl/fl bone marrow. The Mozfl/fl was efficiently recombined in colonies derived from Mozfl/fl;Mx1-cre donors that were poly(I:C) treated 21 months earlier (Figure 5E). One colony with an unrecombined allele (ie, heterozygous) was detected, but there were no Mozfl/fl;Mx1-cre colonies lacking any recombination. Analysis of transplantations using bone marrow from mice 18 months after Moz deletion (transplant series 2) showed that Mozfl/fl alleles were efficiently deleted in Mozfl/fl;Mx1-cre donors, in both competitive and noncompetitive transplantation experiments (supplemental Figure 11).
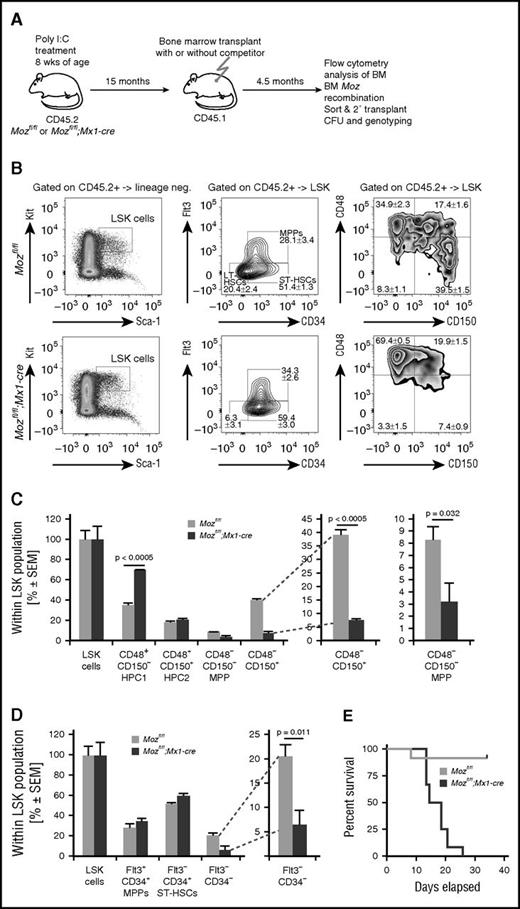
Because we found that the Mozfl/fl;Mx1-cre bone marrow 15 months after Moz deletion (but not 72 hours after Moz deletion) could contribute to all lineages after noncompetitive transplantation in lethally irradiated mice, we analyzed these recipients to determine whether the Mozfl/fl;Mx1-cre repopulating cells had regained the cell surface phenotype typical of HSCs. We quantified the number of LSK cells and further subdivided these into long-term HSCs, short-term HSCs, and multipotent progenitors (MPPs) on the basis of FLT3 and CD34 expression. Likewise, we subdivided the LSK population further on the basis of CD150 and CD48 expression. We found that the recipients of Mozfl/fl;Mx1-cre bone marrow had 3.2-fold fewer LSK-CD34–FLT3– cells and 5.3-fold fewer LSK-CD48–CD150+ cells compared with controls (Figure 6A-D), similar to the donor bone marrow (Figure 4). Similarly, after transplantation of bone marrow from mice 18 months after Moz deletion, we found that the recipients of Mozfl/fl;Mx1-cre bone marrow had 5.8-fold fewer LSK-CD34–FLT3– cells and 4.8-fold fewer LSK-CD48–CD150+ cells compared with controls (supplemental Figure 11). To examine this further we purified the CD45.2 cells from bone marrow of these recipients by FACS and then performed a secondary transplant into CD45.1 recipients. Secondary transplants of controls resulted in survival of the hosts. In contrast, secondary Mozfl/fl;Mx1-cre bone marrow transplants were unable to rescue the lethally irradiated recipients (Figure 6E). The recipients of these cells were moribund within 4 weeks of receiving the transplants, indicating that the primary transplant-derived Mozfl/fl;Mx1-cre bone marrow cells did not contain short-term radio-protective hematopoietic progenitor cells.
Even after successful reconstitution, aged Moz-deleted Mozfl/fl;Mx1-cre bone marrow cells do not exhibit a typical HSC cell surface phenotype. (A) Experimental design. Mice were treated with poly(I:C) and then left undisturbed for 15 months prior to transplantation (same cohort as in Figure 4). Bone marrow stem cell and progenitor subsets were analyzed 4.5 months after transplantation. (B) Flow cytometry plots showing analyses of the LSK-CD34–FLT3– and the LSK-CD48–CD150+ populations in Mozfl/fl and Mozfl/fl;Mx1-cre bone marrow cells after transplantation. (C-D) Quantification of cells with stem cell surface markers by flow cytometry of recipients of Mozfl/fl and Mozfl/fl;Mx1-cre bone marrow cells. Note the reduction in typical LSK-CD34–FLT3– and the LSK-CD48–CD150+ populations after transplantation. (E) CD45.2 (donor) cells were isolated by FACS from primary recipients and 1 × 106 cells transplanted into lethally irradiated secondary recipients; CD45.2 cells from aged Mozfl/fl control mice were able to rescue recipients with high efficiency. In contrast, recipients of CD45.2 Mozfl/fl;Mx1-cre cells rapidly became moribund. PCR genotyping of progenitor colonies from these bone marrow samples are shown in Figure 5E. Data are presented as the mean ± SEM.
Even after successful reconstitution, aged Moz-deleted Mozfl/fl;Mx1-cre bone marrow cells do not exhibit a typical HSC cell surface phenotype. (A) Experimental design. Mice were treated with poly(I:C) and then left undisturbed for 15 months prior to transplantation (same cohort as in Figure 4). Bone marrow stem cell and progenitor subsets were analyzed 4.5 months after transplantation. (B) Flow cytometry plots showing analyses of the LSK-CD34–FLT3– and the LSK-CD48–CD150+ populations in Mozfl/fl and Mozfl/fl;Mx1-cre bone marrow cells after transplantation. (C-D) Quantification of cells with stem cell surface markers by flow cytometry of recipients of Mozfl/fl and Mozfl/fl;Mx1-cre bone marrow cells. Note the reduction in typical LSK-CD34–FLT3– and the LSK-CD48–CD150+ populations after transplantation. (E) CD45.2 (donor) cells were isolated by FACS from primary recipients and 1 × 106 cells transplanted into lethally irradiated secondary recipients; CD45.2 cells from aged Mozfl/fl control mice were able to rescue recipients with high efficiency. In contrast, recipients of CD45.2 Mozfl/fl;Mx1-cre cells rapidly became moribund. PCR genotyping of progenitor colonies from these bone marrow samples are shown in Figure 5E. Data are presented as the mean ± SEM.
Discussion
Our experiments showed that MOZ is essential for the maintenance of adult HSCs. Acute loss of Moz, in the presence of an inflammatory response induced by poly(I:C), results in the loss of long-term repopulating ability in bone marrow cells. This is the classic functional definition of true HSCs.48 The loss of long-term repopulating ability was accompanied by a pronounced reduction of cells carrying 2 different sets of cell surface markers defining a population of cells highly enriched in long-term repopulating HSCs. Furthermore, analysis of the cell cycle of the LSK subpopulations showed that there was a paucity of cells in G0 after Moz deletion. Despite the absence of true HSCs classically defined by repopulation capacity and cell surface makers, we found that the Moz-deleted mice had a normal lifespan and suffered no ill effects. In particular, these mice had a normal hematocrit and normal erythrocyte morphology, showing that the absence of HSCs did not significantly affect the most abundant and most important blood cell type necessary for surviving irradiation.
Moz-deleted cells with the characteristic cell surface phenotype of HSCs were not restored 2 years after Moz deletion, consistent with the rapid and complete loss of HSCs 1 week after Moz deletion, when reconstitution failed, and with the failure of HSCs to develop in Moz germline-null mouse fetuses.15 We followed the development of a cohort of mice after deletion of Moz in the bone marrow for up to 18 months. We anticipated that, if MOZ was necessary to sustain hematopoiesis for the life of the mouse, absence of MOZ function would result in anemia and bone marrow failure. However, we did not expect that induction of cre-recombinase and recombination at the Moz locus would be 100% efficient in all cases, and indeed progenitors with unrecombined Mozfl alleles were detected at low frequency. Therefore, we expected that after a period of time, HSCs that had avoided recombination would take over the bone marrow and the proportion of leukocytes in the blood with deleted alleles would diminish over time. In particular, we expected that Moz-deleted granulocytes would be replaced by granulocytes containing intact Mozfl alleles, because granulocytes are short lived.49 However, this did not occur. Neither did the proportion of nucleated cells with undeleted Mozfl alleles change; these represent long-lived lymphoid cells, mostly B cells but also T cells. This shows that the absence of MOZ, and by implication the absence of HSCs, does not induce any potentially remaining HSCs with intact Moz alleles to proliferate. Interestingly, a recent report has shown that after the death of the majority of the HSCs the long-term HSC (LT-HSC) compartment never fully recovers; however, this does not impede expansion of progenitor cell pools.50 Intriguingly, our results suggest that steady-state hematopoiesis occurs without involvement of HSCs in Moz-deleted mice throughout life.
After acute deletion of Moz, we were unable to detect any competitive repopulating ability; however, after an extended period without Moz, limited repopulating ability emerged in the bone marrow. This suggests that bone marrow cells, although lacking the cell surface markers of classically defined LT-HSCs, may have acquired extended self-renewal capacity. These findings resemble the acquisition of self-renewal by T-cell progenitors in the thymus, which, in the absence of seeding by cells from the bone marrow, develop a self-sustaining lymphoid progenitor that allows continued T-cell production throughout the life of the host.51
The repopulating characteristics of Moz-deleted bone marrow are suggestive of progenitors with extended self-renewal rather than a true multilineage stem cell. First, we found greater myeloid repopulating ability than lymphoid, and second, these cells were unable to compete effectively with wild-type bone marrow–derived cells and were unable to repopulate the hematopoietic system of lethally irradiated recipients after a second round of transplantation. Because myeloid cells have short half-lives,49 the myeloid cells we observe 6 months after transplantation must have arisen from engrafted progenitors that have a high proliferative capacity. We confirmed that the cells supporting steady-state myelopoiesis immediately after deletion of Moz, as well as after 4 months, and in the bone marrow of recipients 6 months after transplantation were derived from Moz-deleted progenitors, by genotyping colony forming, ie, clonal, lineage committed progenitors. In competitive transplants, we found significant levels of myeloid reconstitution, but low levels of lymphoid reconstitution. In part, this may be due to a loss of lymphoid, and enhanced myeloid, reconstituting ability in old mice.11,52,53 However, when the same bone marrow was used in noncompetitive transplant experiments, we found much higher levels of lymphoid reconstitution. This suggests that the cells hematopoiesis in Moz-deleted bone marrow may have features in common with a myeloid-biased progenitor that retains some multilineage differentiation capacity.
Recent studies investigating hematopoiesis through in vivo labeling without transplantation suggest that long-lived progenitors support steady-state hematopoiesis. The majority of these do not have multilineage potential and have limited engraftment capability, but nevertheless can support granulopoiesis for months.6 Furthermore, clonal analyses have identified primitive cells with myeloid-biased differentiation potential, and some of these cells have the ability to sustain granulopoiesis in recipient animals,54,55 although when challenged with competitor cells, myeloid-biased or restricted progenitors are outcompeted.54 In vivo labeling experiments have shown that under normal conditions, myeloid progenitors have far greater capacity for self-renewal than previously suspected.5-7 Our results are consistent with the existence of myeloid-biased progenitors that are able to support hematopoiesis beyond the normal lifespan of a mouse.
Together, our results show that MOZ is essential for the continued presence of normal numbers of HSCs defined by the cell surface markers LSK-CD48−CD150+ and LSK-CD34−Flt3− in adult bone marrow, as well as being required for their formation during embryogenesis. Loss of MOZ function also results in loss of the quiescent population within the stem cell multipotent progenitor compartments. In the absence of classically defined HSCs, mice suffer no adverse effects and we find evidence that steady-state hematopoiesis can be supported for the normal life span of a mouse without the involvement of HSCs assayed in transplantation experiments.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank R. Cobb, N. L. Downer, L. Di Rago, F. Dabrowski, B. Williams, D. Cardozo, and the Walter and Eliza Hall Institute FACS laboratory for excellent technical support.
This work was funded by National Health and Medical Research Council (NHMRC) project grant 1030704; NHMRC research fellowships (1003435 [T.T.] and 575512 [A.K.V.]); Australian Stem Cell Centre grant P-046; the Australian Stem Cell Centre: Adult Stem Cell Program; Independent Research Institutes Infrastructure Support Scheme from the Australian Government’s NHMRC, the Australia Cancer Research Fund; and a Victorian State Government Operational Infrastructure Support Grant.
Authorship
Contribution: B.N.S., Y.Y., J.S., H.M.M., S.K.N., S.C., R.B., D.M., and T.T. carried out experiments; B.N.S., T.T., and A.K.V. analyzed data; T.T. conceived and initiated the project; and A.K.V. and T.T. jointly supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for B.N.S. is Max Planck Institute for Immunobiology and Epigenetics, Freiburg, Germany.
Correspondence: Tim Thomas, 1G Royal Parade, Melbourne, VIC 3052, Australia; e-mail: tthomas@wehi.edu.au; or Anne K. Voss, 1G Royal Parade, Melbourne, VIC 3052, Australia; e-mail: avoss@wehi.edu.au.
References
Author notes
B.N.S. and Y.Y. contributed equally and are joint first authors.
A.K.V. and T.T. contributed equally and are joint senior authors.

![Figure 3. Loss of HSCs has little effect during the normal lifespan of mice. (A) Experimental design of Moz deletion in situ. (B) Quantification of Mozfl alleles in nucleated cells of the peripheral blood by qPCR from 100 to 558 days after poly(I:C) treatment of Mozfl/fl;Mx1-cre (n = 5) and Mozfl/fl (n = 6) control mice. No significant change in the level of Moz-deleted alleles was seen over this period (P = .9775). (C) Peripheral blood analysis of mice 18 months after poly(I:C) treatment of (n = 9) Mozfl/fl;Mx1-cre and (n = 9) Mozfl/fl control mice (complete analysis; supplemental Figure 4). The erythrocyte (RBC) and neutrophil counts were normal. Note the reduction in leukocytes (white blood cells [WBCs]), due to a reduction in lymphocytes (predominantly B cells; supplemental Figure 6). (D) Experimental design of chimeric transplantation first and then Moz deletion 90 days later. The bone marrow from each donor Mozfl/fl;Mx1-cre (n = 3) and control Mozfl/fl (n = 3) mouse was transplanted into 3 recipients together with wild-type CD45.1 bone marrow, as indicated. (E) Percentage of CD45.2-positive Mozfl/fl;Mx1-cre, and Mozfl/fl control cells in peripheral blood changes over time in the presence of wild-type competitors. Data are presented as the mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/19/10.1182_blood-2015-10-676072/4/m_2307f3.jpeg?Expires=1765896887&Signature=fluRQiqxVqPDS7lWZVG1F2MDO2fWa2iegv-eIsxgn08X~G6AeQ7djq4OQb6HZLNo3X6CDzECehBtKJlwywogN6APvbeVIwY9Dw-RVX~ccJmxl9i9e6byDT6xmBRHNvjVcn-l0OG2ykvlRk3-kEEB-JzhflDYeFhlRFnrgff~uf5BSVK1eVxHxBlM860CJ2RCZFMqANeQUXHpJ14PZiYhDte9L3O56ArXi~qnAJhcJg78sejkFoN0AoApysZU-~481wXIKm0jvL4I72h34somx8RF-EwLLfZO3bFHOym9o0tSoHTCalgnmMcuc6hwxRhA3egKC6I0TVq3hsLN6wZErA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)