Key Points
FMT was safely performed in SCT patients, with 3 complete responses and 1 partial response.
Temporal microbiota dynamics seem linked to gut condition and effector regulatory T cells also increased during response to FMT.
Abstract
Increasing evidence indicates that the gut microbiota is closely associated with acute graft-versus-host disease (aGVHD) in stem cell transplantation (SCT). Fecal microbiota transplantation (FMT) could represent an alternative treatment option for aGVHD. However, FMT for SCT patients carries a potential risk of infection by infused microbiota because of the severely immunosuppressed status. We therefore conducted a pilot study to evaluate the safety of FMT in SCT. A total of 4 patients with steroid-resistant (n = 3) or steroid-dependent gut aGVHD (n = 1) received FMT. No severe adverse events attributed to FMT were observed. All patients responded to FMT, with 3 complete responses and 1 partial response. Temporal dynamics of microbiota seemed to be linked to the gut condition of patients and peripheral effector regulatory T cells also increased during response to FMT. FMT was safely performed in our patients and might offer a novel therapeutic option for aGVHD. This trial was registered at the University Hospital Medical Information Network (https://upload.umin.ac.jp/cgi-open-bin/icdr_e/ctr_view.cgi?recptno=R000017575) as #UMIN000015115.
Medscape Continuing Medical Education online
This activity has been planned and implemented through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the American Nurses Credentialing Center (ANCC), the Accreditation Council for Pharmacy Education (ACPE), and the Accreditation Council for Continuing Medical Education (ACCME), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2107.
Disclosures
Laurie Barclay, freelance writer and reviewer, Medscape, LLC, owns stock, stock options, or bonds from Pfizer. Associate Editor Robert Zeiser and the authors declare no competing financial interests.
Learning Objectives
Determine the safety of fecal microbiota transplantation (FMT) for treating acute graft-versus-host disease (aGVHD) of the gut after stem cell transplantation (SCT).
Determine the efficacy of FMT for treating gut aGVHD after SCT.
Assess potential mechanisms underlying FMT as a potential treatment for gut aGVHD after SCT.
Release date: October 20, 2016; October 20, 2017
Introduction
Although allogeneic stem cell transplantation (allo-SCT) is a curative treatment of various hematological diseases, acute graft-versus-host disease (aGVHD) represents a major cause of morbidity and mortality. Glucocorticoids are used as the first-line therapy for aGVHD, but only about half of patients respond1 and no second-line treatment has yet been established.
The gut microbiota and its metabolites have been reported to play pivotal roles in intestinal inflammation and the immune system.2,3 Also in allo-SCT, increasing evidence indicates that the gut microbiota is closely associated with aGVHD.4-10 Fecal microbiota transplantation (FMT) refers to infusion of a fecal suspension from a healthy donor into the gastrointestinal tract of a patient to restore a healthy microbiota and cure disease. Manipulation of the intestinal microbiota by FMT may influence the immune system and improve immune-mediated enteritis such as gut aGVHD. However, FMT carries a potential risk of infection by the infused microbiota for SCT recipients. We therefore conducted this pilot study to evaluate the safety of FMT for treating steroid-resistant or steroid-dependent gut aGVHD.
Study design
Four patients with gut aGVHD (steroid-resistant, n = 3; steroid-dependent, n = 1) underwent this pilot study (Table 111 ).
Patient characteristics, AEs, and response
| . | Case 1 . | Case 2 . | Case 3 . | Case 4 . |
|---|---|---|---|---|
| Age, y/Sex | 64/Female | 44/Female | 48/Male | 42/Male |
| Diagnosis | AML | AML with 3q26.2 abn | MK-AML | AML-MRC |
| Indication for FMT | Resistant | Resistant | Dependent | Resistant |
| GVHD stage (overall) | ||||
| Gut | 1 | 4 | 1 | 2* |
| Skin | 0 | 0 | 0 | 0 |
| Liver | 0 | 3 | 3 | 1 |
| GVHD grade (overall) | II | III | II | IV† |
| GVHD stage at start of FMT | ||||
| Gut | 1 | 4 | 1 | 2* |
| Skin | 0 | 0 | 0 | 0 |
| Liver | 0 | 0 | 0 | 1 |
| GVHD grade at start of FMT | II | III | II | IV† |
| Initial treatment dose of steroid | 2 mg/kg mPSL | 2 mg/kg mPSL | >2 mg/kg mPSL | 1-2 mg/kg mPSL |
| Dose of steroid at start of FMT | 1 mg/kg mPSL | 1 mg/kg mPSL | >2 mg/kg mPSL | 2 mg/kg mPSL |
| Treatment of GVHD other than systemic steroid | FK, beclomethasone | FK, beclomethasone, octreotide, loperamide, fentanyl | FK, beclomethasone | FK, beclomethasone, octreotide |
| Infectious complications and treatment at start of FMT | ||||
| Clostridium difficile toxin | — | — | — | — |
| Comorbid infection | CMV antigenemia | IPA CMV retinitis | IPA | Sepsis (catheter infection) CMV enteritis |
| Antibiotics | ST, TAZ/PIPC | LVFX | CFPM + VCM | CFPM |
| Cessation of antibiotics | Yes (TAZ/PIPC) | Yes | Yes | No |
| Antifungals | MCFG | VRCZ | L-AmphB | MCFG |
| Antivirals | Foscarnet | Ganciclovir (intraocular) Foscarnet | Aciclovir | Foscarnet |
| AEs (grade) | ||||
| First FMT | Abdominal pain (1) | Belch (1) | Diarrhea (1) | Hypoxia (2) |
| Pharyngolaryngeal pain (1) | Anemia (2→3)‡ | Delirium (1) | ||
| Diarrhea (2) | Thrombocytopenia (3→4)‡ | Lower GI hemorrhage (1) | ||
| Hypokalemia (L-AmphB induced) (2) | Hypothyroidism (1) | |||
| γGTP↑ (1→2) | ||||
| Second FMT | Abdominal pain (1) | Abdominal pain (1) | NA | Abdominal pain (1) |
| Pharyngolaryngeal pain (1) | Pharyngolaryngeal pain (1) | Fever (1)‡ | ||
| Nausea (1) | Diarrhea (2) | Blood bilirubin increased (1→3) | ||
| γGTP↑ (2→3) | ||||
| PAF (1) | ||||
| TA-TMA (2)§ | ||||
| Response|| | Complete response | Complete response | Complete response | Partial response |
| . | Case 1 . | Case 2 . | Case 3 . | Case 4 . |
|---|---|---|---|---|
| Age, y/Sex | 64/Female | 44/Female | 48/Male | 42/Male |
| Diagnosis | AML | AML with 3q26.2 abn | MK-AML | AML-MRC |
| Indication for FMT | Resistant | Resistant | Dependent | Resistant |
| GVHD stage (overall) | ||||
| Gut | 1 | 4 | 1 | 2* |
| Skin | 0 | 0 | 0 | 0 |
| Liver | 0 | 3 | 3 | 1 |
| GVHD grade (overall) | II | III | II | IV† |
| GVHD stage at start of FMT | ||||
| Gut | 1 | 4 | 1 | 2* |
| Skin | 0 | 0 | 0 | 0 |
| Liver | 0 | 0 | 0 | 1 |
| GVHD grade at start of FMT | II | III | II | IV† |
| Initial treatment dose of steroid | 2 mg/kg mPSL | 2 mg/kg mPSL | >2 mg/kg mPSL | 1-2 mg/kg mPSL |
| Dose of steroid at start of FMT | 1 mg/kg mPSL | 1 mg/kg mPSL | >2 mg/kg mPSL | 2 mg/kg mPSL |
| Treatment of GVHD other than systemic steroid | FK, beclomethasone | FK, beclomethasone, octreotide, loperamide, fentanyl | FK, beclomethasone | FK, beclomethasone, octreotide |
| Infectious complications and treatment at start of FMT | ||||
| Clostridium difficile toxin | — | — | — | — |
| Comorbid infection | CMV antigenemia | IPA CMV retinitis | IPA | Sepsis (catheter infection) CMV enteritis |
| Antibiotics | ST, TAZ/PIPC | LVFX | CFPM + VCM | CFPM |
| Cessation of antibiotics | Yes (TAZ/PIPC) | Yes | Yes | No |
| Antifungals | MCFG | VRCZ | L-AmphB | MCFG |
| Antivirals | Foscarnet | Ganciclovir (intraocular) Foscarnet | Aciclovir | Foscarnet |
| AEs (grade) | ||||
| First FMT | Abdominal pain (1) | Belch (1) | Diarrhea (1) | Hypoxia (2) |
| Pharyngolaryngeal pain (1) | Anemia (2→3)‡ | Delirium (1) | ||
| Diarrhea (2) | Thrombocytopenia (3→4)‡ | Lower GI hemorrhage (1) | ||
| Hypokalemia (L-AmphB induced) (2) | Hypothyroidism (1) | |||
| γGTP↑ (1→2) | ||||
| Second FMT | Abdominal pain (1) | Abdominal pain (1) | NA | Abdominal pain (1) |
| Pharyngolaryngeal pain (1) | Pharyngolaryngeal pain (1) | Fever (1)‡ | ||
| Nausea (1) | Diarrhea (2) | Blood bilirubin increased (1→3) | ||
| γGTP↑ (2→3) | ||||
| PAF (1) | ||||
| TA-TMA (2)§ | ||||
| Response|| | Complete response | Complete response | Complete response | Partial response |
All AEs that were obviously related to FMT (underlined) were mild and transient.
AML, acute myeloid leukemia; AML-MRC, AML with myelodysplasia-related changes; AML with 3q26.2 abn, AML with 3q26 abnormality; CFPM, cefepime; CMV, cytomegalovirus; FK, tacrolimus; GI, gastrointestinal; GTP, guanosine triphosphate; IPA, invasive pulmonary aspergillosis; L-AmphB, liposomal amphotericin B; LVFX, levofloxacin; MCFG, micafungin; MK-AML, AML with monosomal karyotype; NA, not applicable; ST, sulfamethoxazole/trimethoprim; TAZ/PIPC, tazobactam/piperacillin; TA-TMA, transplant-associated thrombotic microangiopathy; VCM, vancomycin; VRCZ, voriconazole.
Downgraded one stage because of CMV enteritis.
Graded as IV because of extremely poor performance status.
Recovered in 1 day.
TA-TMA was graded using the common toxicity criteria proposed by Ho et al.11
Response of FMT was evaluated 28 days after last infusion (cases 1-3) or as maximum response before rituximab administration (case 4).
The patient’s spouse or a relative who passed the screening for transmissible diseases could be a candidate for FMT donor. The maximum number of treatments was 2 and all adverse events (AEs) that first arose or progressed within 1 week after each infusion were evaluated in terms of the safety of FMT. Response to FMT was evaluated 28 days after the final FMT (cases 1-3) or at the time of maximum response (case 4).
Microbial analyses were performed using 16 ribosomal RNA gene sequencing. For immunological assays, peripheral mononuclear cells were isolated before and after FMT, and analyzed by flow cytometry (detailed information about the study protocol is provided in supplemental Methods, available on the Blood Web site).
Results and discussion
The underlying disease in all patients was acute myeloid leukemia (Table 1). Case 3 was diagnosed with late-onset aGVHD. The first FMT was performed at a median of 92 days after SCT (60-174 days). All patients received the first FMT while receiving methylprednisolone (mPSL) at ≥1 mg/kg and showed comorbid infectious complications at the start of FMT (Table 1).
Median feces volume was 126 g (34-307 g) and the final volume of the fecal suspension was 180 to 230 mL. Fecal suspensions were administered over 4 to 8 minutes via a nasoduodenal tube. FMTs were performed at a median of 6 hours (2.75-9 hours) after feces collection (supplemental Table 1). All AEs that were obviously related to FMT were mild and transient (underlined in Table 1). Case 4 developed AEs such as hypoxia, paroxysmal atrial fibrillation (PAF), lower gastrointestinal bleeding, cholestatic liver damage, and transplant-associated thrombotic microangiopathy. In addition, case 4 also developed fever 2 days after the second FMT. The possibility of an association between FMT and these AEs could not be completely ruled out. Indeed, gastrointestinal bleeding and PAF may be induced by FMT-related complications such as mechanical mucosal damage by tube insertion, or mental discomfort. However, the patient presented with fresh blood in the stool, not tarry stool, and PAF occurred 4 days after the second FMT. Thus, it seemed more plausible that various underlying conditions, such as poor performance status, hypoalbuminemia (≤2 g/dL), severe cytopenias, use of various drugs, or Epstein-Barr virus reactivation, contributed to the development of these AEs. A febrile episode after the second FMT resolved within 1 day and no pathogens were detected. These results thus suggest that normal microbiota12,13 might be administered safely in SCT patients (for the detailed clinical course of each patient, please see supplemental Results and discussion).
With regard to response, FMT was effective in all patients, with complete response in 3 patients and partial response in 1 case, and improvement of gastrointestinal symptoms was observed within several days in the steroid-resistant cases. Moreover, the steroid dose was able to be successfully reduced by more than half (mean, 69% reduction) compared with that before FMT in cases 1 to 3 (supplemental Figure 1).
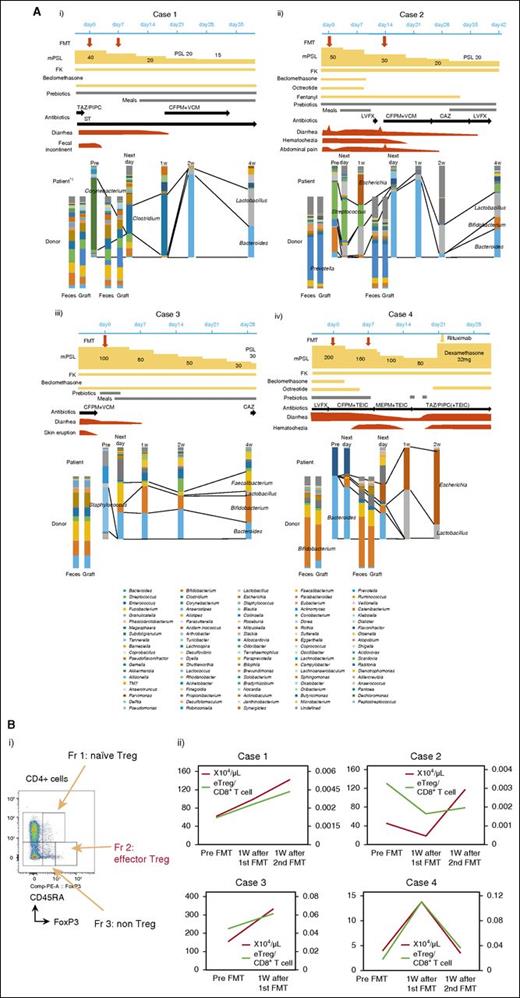
Clinical courses and temporal microbiota dynamics in each patient are shown in Figure 1A. Case 1 showed favorable recovery from gastrointestinal symptoms after FMT and the final microbiota composition was dominated by Lactobacillus and Bacteroides (Figure 1Ai). In case 2, improvement of gastrointestinal symptoms after the first FMT was transient and minimal, but gradually improved after the second FMT. Streptococcus decreased after FMT and this became more prominent after the second FMT. The final microbiota composition was dominated by Bacteroides, Lactobacillus, and Bifidobacterium (Figure 1Aii). In case 3, gastrointestinal symptoms remitted after reescalating mPSL and were not exacerbated. The microbiota after FMT mainly comprised Bacteroides, Bifidobacterium, and Faecalibacterium, and this composition was maintained during the study period (Figure 1Aiii). In case 4, gastrointestinal symptoms gradually improved after 2 courses of FMT, but eventually flared after rapid reduction of mPSL and tacrolimus for transplant-associated thrombotic microangiopathy (13 days after the second FMT). This patient’s feces were eventually occupied by Escherichia at the time of recurrence of gut aGVHD (Figure 1Aiv). Temporal microbiota dynamics thus seemed to be linked to the gut condition of the patient. Beneficial bacteria such as Bacteroides,5 Lactobacillus,8 Bifidobacterium, and Faecalibacterium14 were dominant in cases 1 to 3,15 whereas Escherichia, which has been strongly correlated with GVHD in a mouse model,9 was increased at the recurrence of aGVHD in case 4. The number of operational taxonomic units and diversity of the intestinal microbiota did not fully recover after FMT, even with apparent clinical response (supplemental Figure 2). These results indicate that full recovery of the microbiota might not be indispensable for response to FMT. Indeed, in most SCT patients, the operational taxonomic unit count was very low compared with that in healthy volunteers, even in patients without aGVHD (K. Kakihana and N.D., unpublished data). Although antibiotics had to be resumed in cases 1 and 2, these patients responded to FMT and did not show exacerbation after restarting antibiotics (Figure 1A). Reduced activity against intestinal anaerobic bacteria of the antibiotics used might have contributed to conserve the response to FMT.16 Furthermore, FMT could be performed without exacerbating comorbid infections in any patients. FMT thus may not negatively affect immunity against infection. Regulatory T cells (Tregs) have been reported as a prognostic cellular biomarker for aGVHD17,18 and the number of peripheral effector Tregs (eTregs; Figure 1Bi),19 which have been reported as terminally differentiated and highly suppressive,20 increased during responding to FMT. The eTreg/CD8+ T-cell ratio showed a similar trend in most cases (Figure 1Bii). Similar results were obtained for overall FoxP3+CD4+ T cells (supplemental Figure 3). Although somewhat conflicting, our results indicate that FMT might shift the systemic allogeneic immune response to an anti-inflammatory state by changing the intestinal microbiota and might be effective against other forms of aGVHD. Indeed, the intestinal microbiota has been reported to be associated with the entire spectrum of aGVHD.4,5,10
Components of microbiota and immunological assay. (A) Temporal dynamics of the microbiota (at the genus level) and clinical course in each patient: (i) case 1, (ii) case 2, (iii) case 3, and (iv) case 4. *1: Data from the day after first FMT could not be obtained because of the lack of fecal sample. (B) (i) Subpopulation of Tregs. Tregs can be dissected into 3 subpopulations by expression levels of FoxP3, CD45RA. FoxP3loCD45RA+ cells (fraction 1), designated as naive Tregs, which differentiate into eTregs under antigenic stimulation; FoxP3hiCD45RA− cells (fraction 2), designated eTregs, which are terminally differentiated and highly suppressive; and FoxP3loCD45RA− non-Tregs (fraction 3), which do not possess suppressive activity, but secrete proinflammatory cytokines.20 (ii) The absolute number of eTregs (red lines) and the eTreg/CD8+ T-cell ratio (green lines) in peripheral blood of each patient. CAZ, ceftazidime; CFPM, cefepime; FK, tacrolimus; Fr, fraction; LVFX, levofloxacin; MEPM, meropenem; PSL, prednisolone; ST, sulfamethoxazole/trimethoprim; TAZ/PIPC, tazobactam/piperacillin; TEIC, teicoplanin; VCM, vancomycin.
Components of microbiota and immunological assay. (A) Temporal dynamics of the microbiota (at the genus level) and clinical course in each patient: (i) case 1, (ii) case 2, (iii) case 3, and (iv) case 4. *1: Data from the day after first FMT could not be obtained because of the lack of fecal sample. (B) (i) Subpopulation of Tregs. Tregs can be dissected into 3 subpopulations by expression levels of FoxP3, CD45RA. FoxP3loCD45RA+ cells (fraction 1), designated as naive Tregs, which differentiate into eTregs under antigenic stimulation; FoxP3hiCD45RA− cells (fraction 2), designated eTregs, which are terminally differentiated and highly suppressive; and FoxP3loCD45RA− non-Tregs (fraction 3), which do not possess suppressive activity, but secrete proinflammatory cytokines.20 (ii) The absolute number of eTregs (red lines) and the eTreg/CD8+ T-cell ratio (green lines) in peripheral blood of each patient. CAZ, ceftazidime; CFPM, cefepime; FK, tacrolimus; Fr, fraction; LVFX, levofloxacin; MEPM, meropenem; PSL, prednisolone; ST, sulfamethoxazole/trimethoprim; TAZ/PIPC, tazobactam/piperacillin; TEIC, teicoplanin; VCM, vancomycin.
In summary, FMT was safely performed in SCT patients and offers promise as a potential treatment option for aGVHD. Further evaluation to confirm the safety and efficacy of FMT for aGVHD is warranted. Despite the very small number of patients, our results are highly suggestive for elucidating the associations between microbiota and human immunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are very grateful to the patients and FMT donors who participated in this study. The authors thank Noritaka Sekiya for his professional advice regarding antibiotic use and donor screening.
This work was supported by Grants-in-Aid for Scientific Research (B) (no. 26290054) (H.N.) and for challenging Exploratory Research (no. 16K15551) (H.N.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by The National Cancer Center Research and Development Fund (no. 28-A-7) (H.N.).
Authorship
Contribution: K. Kakihana, Y.N., T.T., and K.O. designed the study; K. Kakihana, K. Koizumi, G.K., Y.N., S.I., K.Y., and Y.H. performed FMT and collected samples; S.S., N.D., I.M., H.M., Y.F., D.S., H.N., W.S., M.H., K. Kakihana, and K.H. carried out research and analyzed the data; and K. Kakihana, Y.F., and W.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kazuhiko Kakihana, Hematology Division, Tokyo Metropolitan Cancer and Infectious diseases Center, Komagome Hospital, 3-18-22 Honkomagome, Bunkyo-ku, Tokyo 113-8677, Japan; e-mail: kakihana@cick.jp.
References
Author notes
K. Kakihana, Y.F., and W.S. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal