Abstract
This article summarizes our approach to the management of children and adults with primary immune thrombocytopenia (ITP) who do not respond to, cannot tolerate, or are unwilling to undergo splenectomy. We begin with a critical reassessment of the diagnosis and a deliberate attempt to exclude nonautoimmune causes of thrombocytopenia and secondary ITP. For patients in whom the diagnosis is affirmed, we consider observation without treatment. Observation is appropriate for most asymptomatic patients with a platelet count of 20 to 30 × 109/L or higher. We use a tiered approach to treat patients who require therapy to increase the platelet count. Tier 1 options (rituximab, thrombopoietin receptor agonists, low-dose corticosteroids) have a relatively favorable therapeutic index. We exhaust all Tier 1 options before proceeding to Tier 2, which comprises a host of immunosuppressive agents with relatively lower response rates and/or greater toxicity. We often prescribe Tier 2 drugs not alone but in combination with a Tier 1 or a second Tier 2 drug with a different mechanism of action. We reserve Tier 3 strategies, which are of uncertain benefit and/or high toxicity with little supporting evidence, for the rare patient with serious bleeding who does not respond to Tier 1 and Tier 2 therapies.
Case 1
A 10-year-old male was diagnosed with primary immune thrombocytopenia (ITP) 5 months ago when he presented with epistaxis, petechiae, bruising, and a platelet count of 5 × 109/L. He responded transiently to intravenous immunoglobulin G (IgG), but epistaxis recurred 2 weeks later. He subsequently received a short course of oral corticosteroids to which he had a temporary response in platelet count and cessation of epistaxis. Since discontinuing corticosteroids, he has had only occasional bruising and petechiae. His platelet count is currently 13 × 109/L. He states that ITP is not interfering with activities.
Introduction
ITP treatment may be conceptually divided into rescue therapy and maintenance therapy. The objective of rescue therapy is a swift rise in platelet count in a patient with active hemorrhage, a high risk for bleeding, or need for a critical procedure. In selecting rescue therapy, a premium is placed on rapidity of response with relatively less regard for durability of response, patient convenience, or safety and tolerability with long-term use. Maintenance therapy, in contrast, is given with the goal of achieving a sustained platelet response while minimizing short- and long-term treatment-related toxicity. Goals and standard treatment options for rescue and maintenance therapy are summarized in Table 1.
Goals and standard treatment options for rescue and maintenance therapy
| . | Rescue therapy . | Maintenance therapy . |
|---|---|---|
| Goals of treatment | Rapid platelet response | Durable platelet response |
| Short-term safety | Long-term safety and tolerability | |
| Patient convenience | ||
| Desired time to response | Hours to days | Days to weeks |
| Standard treatment options | Corticosteroids | Splenectomy |
| IVIG | ||
| Anti-D* |
| . | Rescue therapy . | Maintenance therapy . |
|---|---|---|
| Goals of treatment | Rapid platelet response | Durable platelet response |
| Short-term safety | Long-term safety and tolerability | |
| Patient convenience | ||
| Desired time to response | Hours to days | Days to weeks |
| Standard treatment options | Corticosteroids | Splenectomy |
| IVIG | ||
| Anti-D* |
IVIG, intravenous immunoglobulin G.
Indicated only in Rh(D)-positive, nonsplenectomized patients.
Management of the patient with ITP whose platelet count does not respond to standard therapy (Table 1) can be frustrating and terrifying for clinicians and patients, particularly if there is active bleeding or severe thrombocytopenia. The purpose of this article is to summarize our approach to the management of children and adults with primary ITP who do not respond to or cannot tolerate standard maintenance therapy (ie, splenectomy). Much of the evidence in this area is limited to case reports and uncontrolled series.1 There are few direct comparisons of different treatment strategies. Where evidence is available, it is cited. Suggestions are otherwise based on our experience and opinions.
What is refractory ITP?
An International Working Group (IWG) defined refractory ITP as disease that does not respond to or relapses after splenectomy and that requires treatment to reduce the risk of clinically significant bleeding.2 The American Society of Hematology ITP Guidelines endorsed this definition as a means of identifying the most severely affected patients.3 Given that splenectomy remains the surest means of “curing” ITP with long-term complete platelet response rates of 60% to 70%,4,5 this characterization remains pertinent.
Nevertheless, several limitations of the IWG definition2 must be acknowledged. First, it may not be applicable to certain patient populations such as children, those with significant comorbidities, and those unwilling to undergo splenectomy. In these patients, avoidance of splenectomy may be desirable or even necessary. Indeed, the IWG acknowledged that it was unable to achieve consensus on the definition of refractory disease in children.2 Second, the IWG definition stipulates that treatment be deemed necessary for bleeding symptoms or presumed high risk of bleeding. It is conceivable that, for some patients, the indication for treatment after splenectomy failure might relate to something other than bleeding symptoms such as improvement in health-related quality of life (HRQoL).6,7 HRQoL is a multidimensional construct that focuses on the impact of health on physical, mental, social, and emotional functioning.8 Third, the IWG classification does not include refractoriness to rescue therapy.
Herein, we use a broader definition of refractoriness than the IWG does. Regarding patients refractory to maintenance therapy, we include not only patients who require treatment to mitigate bleeding risk after splenectomy but also patients who are unable or disinclined to undergo splenectomy and patients in whom the primary objective of treatment is improvement in HRQoL.
ITP refractory to maintenance therapy
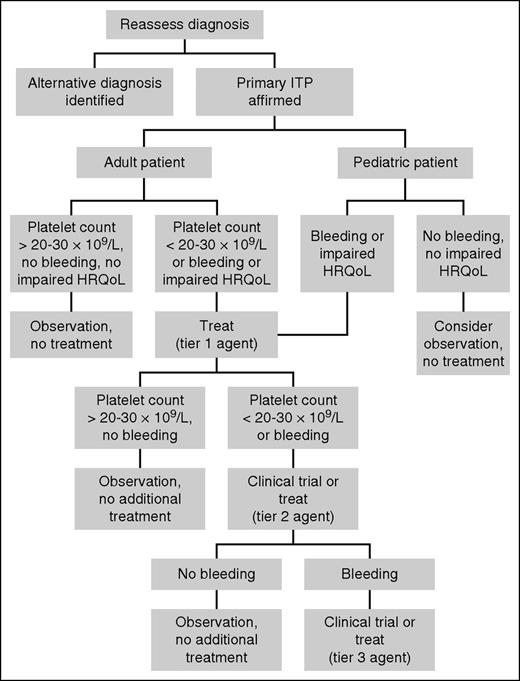
Figure 1 illustrates our approach to the patient who is refractory to or ineligible for splenectomy. Key features include reassessment of the diagnosis, consideration of observation without treatment, a 3-tiered approach to treatment, and the use of combination therapy.
Approach to the patient with refractory ITP. We begin by reassessing the diagnosis of ITP and excluding nonautoimmune causes of thrombocytopenia and secondary ITP. After ITP has been affirmed, we consider whether treatment is indicated. Observation alone is appropriate for most adults with a platelet count >20 to 30 × 109/L and no bleeding or impaired HRQoL and for most children without bleeding or impaired HRQoL. For patients who require treatment, we treat with a Tier 1 agent (Table 2). We select an agent based on age, comorbidities, drug availability, cost, and patient preference. In patients who do not respond to or cannot tolerate a Tier 1 agent, we move on to another Tier 1 agent. In patients who have exhausted all options in Tier 1, we consider enrollment in a clinical trial. If a suitable trial is not available, we initiate a Tier 2 agent (Table 3). We often use Tier 2 agents in combination with Tier 1 or other Tier 2 agents with different mechanisms of action. For the rare patient with serious bleeding who does not achieve an acceptable response with Tier 2 agents, we again consider enrollment in a clinical trial or initiation of a Tier 3 treatment (Table 4).
Approach to the patient with refractory ITP. We begin by reassessing the diagnosis of ITP and excluding nonautoimmune causes of thrombocytopenia and secondary ITP. After ITP has been affirmed, we consider whether treatment is indicated. Observation alone is appropriate for most adults with a platelet count >20 to 30 × 109/L and no bleeding or impaired HRQoL and for most children without bleeding or impaired HRQoL. For patients who require treatment, we treat with a Tier 1 agent (Table 2). We select an agent based on age, comorbidities, drug availability, cost, and patient preference. In patients who do not respond to or cannot tolerate a Tier 1 agent, we move on to another Tier 1 agent. In patients who have exhausted all options in Tier 1, we consider enrollment in a clinical trial. If a suitable trial is not available, we initiate a Tier 2 agent (Table 3). We often use Tier 2 agents in combination with Tier 1 or other Tier 2 agents with different mechanisms of action. For the rare patient with serious bleeding who does not achieve an acceptable response with Tier 2 agents, we again consider enrollment in a clinical trial or initiation of a Tier 3 treatment (Table 4).
Reassessment of the diagnosis
There is no gold standard diagnostic test for ITP. The most compelling evidence for the presence of ITP is a platelet response to standard therapy (Table 1). Nonresponsiveness to treatment should therefore prompt reassessment of the diagnosis and a concerted effort to exclude nonautoimmune causes of thrombocytopenia and secondary ITP. The reader is referred elsewhere for a discussion of diagnostic evaluation.3,9,10 It is likewise imperative that a manual platelet count be performed because automated cell counters may misclassify large or agglutinated platelets, leading to an underestimation of the platelet count and potentially inappropriate treatment decisions.
Observation
In accordance with American Society of Hematology guidelines,3 we recommend observation without treatment in most asymptomatic adults after splenectomy with a platelet count of 20 to 30 × 109/L or greater. In a prospective cohort study, patients who maintained a platelet count above this threshold after splenectomy had no bleeding-related deaths. In contrast, patients with a platelet count below 30 × 109/L had a bleeding-related mortality of 36.7%.11 In a separate study of 47 patients who were unable to maintain a platelet count of 100 × 109/L after splenectomy, there were 3 deaths over a median follow-up of 7.5 years. All 3 had a baseline platelet count below 20 × 109/L.12 A platelet count threshold above 30 × 109/L may be preferred in some patients because of their occupation, participation in sports, need for antithrombotic therapy, or control of other symptoms that track with platelet count (eg, fatigue, HRQoL). Conversely, some patients have little or no bleeding at platelet counts well below 30 × 109/L and are more concerned about adverse effects of treatment than bleeding. These individuals may elect to defer therapy at lower platelet counts. This is particularly relevant to children, who have low bleeding risk even in the setting of severe persistent or chronic thrombocytopenia.13 Observation may be appropriate in asymptomatic or minimally symptomatic children, as in Case 1 above, irrespective of the platelet count. We prioritize bleeding symptoms and HRQoL over platelet count in decision-making in this population (Figure 1).
Tiered approach to treatment
For patients with refractory ITP who require therapy to maintain a hemostatic platelet count, numerous treatments are available. We divide these options into 3 tiers based on efficacy, safety, and quality of evidence.14 Treatment options in Tier 1 have a relatively favorable therapeutic index and are supported by reasonably good evidence. Options in Tier 3, in contrast, have a less auspicious efficacy/safety profile and/or a weak evidentiary basis.
There are no direct comparisons of treatment options within a given tier. We therefore individualize choice of treatment on the basis of age, comorbidities, drug availability, cost, and patient preference. We exhaust options in each tier before proceeding to the subsequent tier. When a durable platelet response cannot be attained with monotherapy, we consider combining agents with different mechanisms of action.
Case 1 continued
The child has been managed with observation for the past 3 months and is now 8 months from his diagnosis. He continues to have no bleeding symptoms apart from bruising and petechiae. His platelet count has varied between 10 and 30 × 109/L. Football season will begin shortly and he desires to have a platelet count that is safe for participation.
Tier 1
Low-dose corticosteroids.
Rarely, patients who are highly responsive to corticosteroids at standard rescue therapy doses (eg, prednisone 1 mg/kg/d, dexamethasone 40 mg/d × 4) may be able to sustain hemostatic platelet counts at very low maintenance doses of prednisone (≤5 mg/d).15 Long-term treatment at such doses is generally well tolerated but is not devoid of risk for cumulative toxicities, including weight gain, diabetes mellitus, hypertension, decreased bone mineral density, and cataract formation (Table 2).16 Monitoring for toxicities, including periodic bone density assessment, is indicated. Calcium and vitamin D supplementation and regular weight-bearing exercise are recommended to reduce the risk of osteoporosis.
Tier 1 treatment options
| Drug . | Dose . | Response rate* . | Time to response (wk) . | Selected toxicities . |
|---|---|---|---|---|
| Low-dose prednisone | ≤5 mg orally once per day | <10% | N/A† | Weight gain, hyperglycemia, hypertension, osteoporosis, cataracts |
| Rituximab | 375 mg/m2 IV once per week × 4 (lower doses may be effective) | 60% overall, 40% complete, 20%-25% at 5 y | 1-8 | Infusion reactions, serum sickness, HBV reactivation, PML (rare) |
| Romiplostim | 1-10 μg/kg SC once per week | 80% overall, 40%-50% persistent | 1-4 | Reticulin fibrosis, rebound thrombocytopenia, thrombosis |
| Eltrombopag | 25-75 mg orally once per day | 80% overall, 40%-50% persistent | 1-2 | Reticulin fibrosis, rebound thrombocytopenia, thrombosis, hepatotoxicity |
| Drug . | Dose . | Response rate* . | Time to response (wk) . | Selected toxicities . |
|---|---|---|---|---|
| Low-dose prednisone | ≤5 mg orally once per day | <10% | N/A† | Weight gain, hyperglycemia, hypertension, osteoporosis, cataracts |
| Rituximab | 375 mg/m2 IV once per week × 4 (lower doses may be effective) | 60% overall, 40% complete, 20%-25% at 5 y | 1-8 | Infusion reactions, serum sickness, HBV reactivation, PML (rare) |
| Romiplostim | 1-10 μg/kg SC once per week | 80% overall, 40%-50% persistent | 1-4 | Reticulin fibrosis, rebound thrombocytopenia, thrombosis |
| Eltrombopag | 25-75 mg orally once per day | 80% overall, 40%-50% persistent | 1-2 | Reticulin fibrosis, rebound thrombocytopenia, thrombosis, hepatotoxicity |
HBV, hepatitis B virus; IV, intravenous; N/A, not applicable; PML, progressive multifocal leukoencephalopathy; SC, subcutaneous.
Studies vary in their definition of response.
In patients who are responding to intermediate or high doses of prednisone, we taper to low-dose prednisone. We do not start low-dose prednisone de novo.
Rituximab.
Rituximab is a chimeric monoclonal anti-CD20 antibody that depletes B lymphocytes. Its efficacy was evaluated in a systematic review and meta-analysis collectively involving 19 studies and 313 patients, approximately half of whom had undergone splenectomy. A response (platelet count >50 × 109/L) was achieved in 63% of patients. Median time to response was 5.5 weeks, and median duration of response was 11 months.17 In a long-term observational study of 72 adults and 66 children, the response rate to rituximab at 5 years was 21% and 26%, respectively.18 Published evidence suggests that the likelihood and duration of platelet response are similar in splenectomized and nonsplenectomized patients.17,19 Complete responders who relapse ≥1 year after treatment are likely to respond to retreatment.
The optimal dose of rituximab for ITP treatment has not been established. We use 375 mg/m2 once per week × 4 weeks, but lower doses may be effective (Table 2).20 Rituximab is generally well tolerated. Toxicities include infusion reactions, serum sickness, and prolonged immune suppression. Patients should be screened for hepatitis B before receiving rituximab because of the risk of viral reactivation. We generally avoid rituximab in patients with hepatitis B, although we consider co-administration of antiviral therapy21 in patients who do not respond to or are ineligible for all other Tier 1 options. One case of progressive multifocal leukoencephalopathy has been reported in a patient with ITP treated with rituximab.22 We provide immunizations before beginning treatment because rituximab attenuates response to vaccines for up to 6 months.23
Thrombopoietin receptor agonists.
Thrombopoietin receptor agonists (TRAs) bind to the thrombopoietin receptor and stimulate megakaryocyte maturation and platelet production. Two TRAs, romiplostim and eltrombopag, have been approved by the US Food and Drug Administration. We do not have experience with recombinant human thrombopoietin, which is available in jurisdictions outside the United States.
Romiplostim is given as a once-per-week subcutaneous injection. The starting dose is 1 μg/kg. The dose is increased by 1 μg/kg each week (maximum dose 10 μg/kg) until a platelet count of ≥50 × 109/L is achieved (Table 2). In a randomized, placebo-controlled trial of 63 splenectomized adult patients, the rate of overall response (platelet count ≥50 × 109/L at any time during the study) and durable response (platelet count ≥50 × 109/L for at least 6 of the final 8 weeks of the study) was 79% and 38% in the romiplostim group and 0% and 0% in the placebo group, respectively.24 Romiplostim also reduced bleeding and improved HRQoL. An extension study enrolled 95 splenectomized patients. A platelet response (≥50 × 109/L) was achieved in 90% of the patients and persisted for a median 67% of time on study.25 Thirty-nine patients with prior splenectomy were enrolled in a French compassionate use program. Many of them had also received rituximab and other immunosuppressive agents. Despite refractoriness to previous therapies, 45% met the criteria for long-term response at 2 years.26
Romiplostim is not approved for treatment of children, but preliminary data suggest that it may be effective and safe in this population. In a randomized controlled trial of children (ages 6 months to 17 years), 15 of 17 patients allocated to romiplostim and 0 of 5 assigned to placebo had a platelet response (≥50 × 109/L for 2 consecutive weeks). Of 6 splenectomized children in the romiplostim arm, 4 demonstrated a platelet response. There were no treatment-related serious adverse events.27 In a subsequent open-label extension study, romiplostim maintained platelet responses for more than 4 years without significant toxicity.28 The results of a larger placebo-controlled trial (NCT01444417) that completed enrollment in 2015 have not yet been reported.
Eltrombopag is formulated as a pill to be taken once per day. The starting dose is 50 mg/d (25 mg/d in individuals of East Asian ancestry, those with liver impairment, or those age 1 to 5 years). The dose may be increased to a maximum of 75 mg/d to achieve a platelet count ≥50 × 109/L (Table 2). In a phase III trial, 197 adults were randomly assigned to eltrombopag or placebo for 6 months. Seventy-one of these patients had undergone splenectomy. A durable platelet response (50 to 400 × 109/L for at least 6 of the last 8 weeks on study) was observed in 51% of splenectomized patients assigned to eltrombopag. Eltrombopag was associated with reduced bleeding and concomitant medication usage.29 In a 6-week randomized trial, 114 patients were allocated to eltrombopag or placebo. Among the 45 splenectomized patients in the trial, a platelet response (≥50 × 109/L at 6 weeks) was more common in the eltrombopag arm than in the placebo arm (62.1% vs 15.4%).30 An open-label extension study included 115 splenectomized patients observed for up to 3 years of treatment with eltrombopag. Eighty percent achieved a platelet count ≥50 × 109/L at least once during the study.31 In a real-world retrospective study of 164 Spanish patients taking eltrombopag (104 of whom had undergone splenectomy), 88.5% manifested a response (platelet count ≥30 × 109/L and doubling from baseline).32
Eltrombopag is approved by the Food and Drug Administration for children age 1 year old or older with chronic ITP who have not achieved an appropriate response by using other medications or with splenectomy. Approval was based on the placebo-controlled PETIT trials, which showed that 36% to 40% of eltrombopag-treated children maintained a platelet count of ≥50 × 109/L for the majority of time on study compared with 0% to 3% who received placebo. In the open-label extension studies that followed the randomized trials, 80% of patients achieved a platelet count ≥50 × 109/L on at least one time point during the study. Less than 10% of patients enrolled in the PETIT trials had been previously splenectomized.33,34
The TRAs are generally well tolerated. In a pooled analysis of 14 trials of adults with ITP treated with romiplostim, bone marrow reticulin was observed in 17 of 921 patients.35 In the eltrombopag extension study, 2 of 117 bone marrow biopsies showed moderate to marked reticulin fibrosis.36 Systematic investigations of bone marrow fibrosis have not been undertaken in children. TRAs may increase the incidence of thromboembolism in some populations, but a meta-analysis suggested that these agents do not increase thrombosis in patients with ITP compared with placebo.37 When TRAs are discontinued, the platelet count generally returns to and may temporarily fall below baseline (rebound thrombocytopenia). Some patients experience prolonged platelet responses after discontinuation of therapy.38 Hepatotoxicity may occur with eltrombopag, and monitoring of liver function tests is mandatory.
Case 1 continued
Eltrombopag was initiated to achieve a sufficient platelet count for football season. The platelet count remained between 95 and 200 × 109/L for 4 months. After football season, eltrombopag was discontinued, and the patient maintained a platelet count >150 × 109/L without need for further treatment.
Case 2
A 64-year-old man was diagnosed with primary ITP 18 months ago when he presented with epistaxis, petechiae, and a platelet count of 8 × 109/L. He had a robust response to corticosteroids but relapsed when prednisone was tapered. One year ago, he received rituximab 375 mg/m2 once per week × 4 without response. Six months ago, he underwent laparoscopic splenectomy. Although his platelet count rose briefly after surgery, it subsequently fell to 10 × 109/L. Trials of eltrombopag and then romiplostim produced intermittent spikes in the platelet count but did not lead to a sustained response or permit reduction in the dose of prednisone. He remains dependent on prednisone 20 mg daily to maintain a platelet count of 20 to 30 × 109/L. He complains of insomnia, weight gain, and dysphoria on corticosteroids. A bone density scan shows osteopenia.
Tier 2
If a patient is unable to maintain a hemostatic platelet count with single-agent therapy using the options in Tier 1, we encourage participation in a clinical trial. If a suitable clinical trial is not available, we proceed to Tier 2 agents. We frequently prescribe Tier 2 agents not as monotherapy but in combination with a Tier 1 or another Tier 2 drug. Myriad multiagent regimens exist, but few have been formally studied.39-47 We prefer to combine drugs with different mechanisms of action, which allows for potential synergy between agents. For example, we often use a TRA to boost platelet production in combination with a drug that interferes with platelet clearance (eg, low-dose prednisone or danazol) or autoantibody production (eg, azathioprine or mycophenolate mofetil [MMF]). This approach may induce a more rapid increase in the platelet count than monotherapy, given the delayed time to response of many Tier 2 agents (Table 3).
Tier 2 treatment options
| Drug . | Dose . | Response rate (%)* . | Time to response . | Selected toxicities . |
|---|---|---|---|---|
| 6-mercaptopurine | 50-75 mg/m2 orally once per day | 83 | Not reported | Hepatotoxicity, neutropenia, infection, pancreatitis |
| Azathioprine | 1-2 mg/kg orally once per day (maximum 150 mg/d) | 40-60 | 3-6 mo | Hepatotoxicity, neutropenia, infection, pancreatitis |
| Cyclosporin A | 5-6 mg/kg/d orally divided into 2 doses per day (titrate to blood levels of 100-200 ng/mL) | 30-60 | 3-4 wk | Nephrotoxicity, hypertension, tremor, parathesias, gingival hyperplasia |
| Cyclophosphamide | 0.3-1.0 g/m2 IV repeated once every 2 to 4 weeks × 1 to 3 doses; 50-200 mg orally once per day; after response has been achieved, dose can be tapered to 50 mg | 24-85 | 1-16 wk | Neutropenia, nausea/vomiting, infertility, secondary malignancy |
| Danazol | 50-800 mg/d orally divided into 2 to 4 doses per day | 10-70 | 3-6 mo | Hepatotoxicity, virilization, amenorrhea |
| Dapsone | 75-100 mg orally once per day | 40-75 | 3 wk | Hemolysis (in patients with G6PD deficiency), rash, nausea, methemoglobinuria |
| Mycophenolate mofetil | 250-1000 mg orally twice per day | 11-80 | 4-6 wk | Headache, diarrhea, nausea, anorexia, infection |
| Vinca alkaloids | Vincristine: 1 to 2 mg IV once per week × 3 weeks Vinblastine: 10 mg IV once per week × 3 weeks | 10-75 | 5-7 d | Peripheral neuropathy, vesication at infusion site, constipation, fever, neutropenia |
| Drug . | Dose . | Response rate (%)* . | Time to response . | Selected toxicities . |
|---|---|---|---|---|
| 6-mercaptopurine | 50-75 mg/m2 orally once per day | 83 | Not reported | Hepatotoxicity, neutropenia, infection, pancreatitis |
| Azathioprine | 1-2 mg/kg orally once per day (maximum 150 mg/d) | 40-60 | 3-6 mo | Hepatotoxicity, neutropenia, infection, pancreatitis |
| Cyclosporin A | 5-6 mg/kg/d orally divided into 2 doses per day (titrate to blood levels of 100-200 ng/mL) | 30-60 | 3-4 wk | Nephrotoxicity, hypertension, tremor, parathesias, gingival hyperplasia |
| Cyclophosphamide | 0.3-1.0 g/m2 IV repeated once every 2 to 4 weeks × 1 to 3 doses; 50-200 mg orally once per day; after response has been achieved, dose can be tapered to 50 mg | 24-85 | 1-16 wk | Neutropenia, nausea/vomiting, infertility, secondary malignancy |
| Danazol | 50-800 mg/d orally divided into 2 to 4 doses per day | 10-70 | 3-6 mo | Hepatotoxicity, virilization, amenorrhea |
| Dapsone | 75-100 mg orally once per day | 40-75 | 3 wk | Hemolysis (in patients with G6PD deficiency), rash, nausea, methemoglobinuria |
| Mycophenolate mofetil | 250-1000 mg orally twice per day | 11-80 | 4-6 wk | Headache, diarrhea, nausea, anorexia, infection |
| Vinca alkaloids | Vincristine: 1 to 2 mg IV once per week × 3 weeks Vinblastine: 10 mg IV once per week × 3 weeks | 10-75 | 5-7 d | Peripheral neuropathy, vesication at infusion site, constipation, fever, neutropenia |
G6PD, glucose 6-phosphate dehydrogenase.
Studies vary in their definition of response.
Vinca alkaloids.
Evidence for vinca alkaloids (VAs) is drawn mostly from studies conducted in the 1970s and 1980s, with few reports from the modern era.48-57 Published studies vary in which VAs were used (vincristine vs vinblastine), the dose and number of infusions, use of maintenance dosing, and definition of response. Although initial reports were promising, relatively poor durability of response has dampened enthusiasm for VAs. In an early report of 21 patients, two-thirds achieved a platelet count ≥50 × 109/L.55 Several subsequent studies showed similar response rates. However, later studies found that most responses were transient.48-51,53 In a prospective trial, 35% of patients treated with vincristine achieved a platelet count >100 × 109/L, but a 40% decline in the platelet count was observed as soon as 8 weeks after completion of 3 infusions.51 The fleetingness of response to VAs must be balanced against their toxicities, which include vesication, constipation, and peripheral neuropathy (symptoms generally arise after cumulative vincristine doses of 30 to 50 mg).48,49,51-55 Because patients have a relatively short time to response (5 to 7 days), we consider VAs for those who require a rapid rise in platelet count and do not respond to standard rescue therapy (Table 1). VAs are generally not a good option for induction of long-term remission.
Dapsone.
Dapsone was first discovered as a potential agent for ITP in 1988 when it improved the platelet count in a patient with systemic lupus erythematosus.58 There are no randomized clinical trials of dapsone in ITP. Its use is based on prospective and retrospective cohort studies.59-61 In one of the largest series, 66 patients with chronic ITP were treated with oral dapsone at a dose of 75 to 100 mg. A platelet count >50 × 109/L was achieved in 33 patients (50%), 20 of whom continued to respond for a median duration of 12.5 months.59 In a literature review of dapsone, overall response rates ranged from 40% to 75%.62
Toxicities of dapsone include methemoglobinemia, agranulocytosis, aplastic anemia, hypersensitivity, gastrointestinal complications, and hemolysis in patients with glucose 6-phosphate dehydrogenase (G6PD) deficiency.62 We screen males for G6PD deficiency before beginning dapsone.
Danazol.
Danazol is an attenuated androgen. Response rates vary widely—from 10% to 70%—between studies.63-69 One factor that may account for this variation is heterogeneity in dosing, with some studies investigating low-dose danazol (50 mg orally once per day)64,65 compared with conventional dosing (400 to 800 mg divided into 2 to 4 doses per day).63,66,67,69 The effect of danazol appears to be greatest when patients are able to remain on therapy for a prolonged period. In a series of 96 patients, 7 (70%) of the 10 patients in whom danazol therapy was discontinued relapsed within 6 months. Responses were more durable for patients who remained on therapy for at least 1 year.69
It has been our observation that danazol is more likely to elicit a response in patients who respond well to corticosteroids. Therefore, we use corticosteroid responsiveness as a criterion for selecting danazol. Liver function tests must be monitored regularly. We rarely use danazol in women because of its virilizing effects.
Cyclophosphamide.
Cyclophosphamide may be given intravenously (500 to 1000 mg/m2 once every 3 to 4 weeks × 2 to 3 courses) or orally (1 to 2 mg/kg once per day). Evidence for its use in ITP is limited to 2 early studies, which showed complete or excellent responses in 50% of patients and partial responses in an additional 20% of patients.70,71 Although these response rates compare favorably with other Tier 2 agents, we use cyclophosphamide infrequently because of a paucity of data and the potential for serious long-term sequelae, including malignancy and infertility. Patients receiving cyclophosphamide should drink at least 2 L of fluids per day to prevent hemorrhagic cystitis, and blood counts should be monitored once per week.
Antimetabolites.
The antimetabolites, azathioprine, 6-mercaptopurine, and MMF have been studied in patients with refractory ITP. Azathioprine demonstrated an overall response rate of 64% in a study of 53 adult patients. When the drug was discontinued, 42% remained in remission for a variable follow-up of 7 to 43 months.72 Smaller series also showed favorable results.73,74 One study of 6-mercaptopurine in pediatric autoimmune cytopenias has been published. In that series of 29 patients, there was an 83% response rate, yet only 4 children (2 with ITP) remained in remission without additional immunosuppressive therapy.75 Major toxicities of azathioprine and 6-mercaptopurine include myelosuppression, hepatotoxicity, and pancreatitis.
A greater number of studies have investigated MMF.76-82 We begin MMF at a dose of 500 mg orally twice per day and increase the dose to 1000 to 1500 mg twice per day after 2 weeks. Protocols that use this approach have demonstrated overall response rates of 50% to 60%.76-81 Durability of response after discontinuation of therapy is variable.77,79,82 MMF is generally well tolerated; principal adverse effects include headache and gastrointestinal symptoms.
Cyclosporin A.
Several small studies highlight the effectiveness of cyclosporine A for the management of refractory ITP.83-85 In the largest study of 20 patients, response rates were 50% to 60%. Adverse effects, which include hypertension and nephrotoxicity, led to discontinuation in 6 patients (30%).85 A study of 14 children reported a response rate of ∼30% and 1 life-threatening fungal infection.84 Patients require regular monitoring of blood pressure, renal function, and drug levels.
Case 2 continued
Danazol was initiated at a dose of 200 mg twice per day and increased to 400 mg twice per day while the patient remained on romiplostim 10 μg/kg once per week and prednisone 20 mg once per day. The patient tolerated danazol well without transaminitis or other toxicities. Three months after beginning danazol, his platelet count was 60 × 109/L. Over the ensuing 6 weeks, prednisone was tapered off. The patient remains on danazol and romiplostim with a platelet count of 40 to 50 × 109/L.
Tier 3
In the rare patient with serious bleeding who does not respond to or is ineligible for all Tier 1 and Tier 2 treatment options, including combination therapy, we recommend enrollment in a clinical trial. If a suitable trial is not available, we consider Tier 3 approaches (Figure 1). Tier 3 options are characterized by low response rates and/or high toxicity. Many studies of Tier 3 agents are small and include a mix of patients with newly diagnosed and intractable disease, which clouds the interpretation of response rates in refractory ITP. Because Tier 3 treatment options have a weak evidentiary basis and unfavorable therapeutic index, we reserve them for severely refractory patients with a history of serious or life-threatening bleeding. Table 4 summarizes these approaches, highlighting evidence from studies of 10 or more patients.86-100
Tier 3 treatment options
| Treatment . | Reference . | Dose . | Response rate (%) . | Selected toxicities . |
|---|---|---|---|---|
| ATRA | 86 | 10 mg orally 3 times per day | 29 | Retinoic acid syndrome, flu-like symptoms, musculoskeletal pain, nausea/vomiting, peripheral neuropathy |
| Autologous HSCT | 87 | Cyclophosphamide 50 mg/kg IV once per day × 4 days (conditioning) | 43 | Neutropenic fever, infection |
| Colchicine | 88 | 1.2 g orally once per day | 21 | Agranulocytosis, neuritis, diarrhea, nausea/vomiting |
| Interferon α | 89-92 | Various | 0-36 | Neutropenia, fever, influenza-like symptoms, hepatotoxicity |
| Plasma exchange | 93-95 | One plasma volume exchange once per day × 1 to 8 days | 29-80 | Hypocalcemia, anaphylactoid reactions |
| Protein A immunoadsorption | 96 | Average of 6 treatments (0.25 to 2.0 L plasma per treatment) over 2 to 3 weeks | 21 | Hypersensitivity reactions, pain, nausea/vomiting |
| Cardiopulmonary complications | ||||
| Vitamin C | 97-100 | 2 g orally once per day | 0-82 | Dsypepsia, nausea/vomiting |
| Treatment . | Reference . | Dose . | Response rate (%) . | Selected toxicities . |
|---|---|---|---|---|
| ATRA | 86 | 10 mg orally 3 times per day | 29 | Retinoic acid syndrome, flu-like symptoms, musculoskeletal pain, nausea/vomiting, peripheral neuropathy |
| Autologous HSCT | 87 | Cyclophosphamide 50 mg/kg IV once per day × 4 days (conditioning) | 43 | Neutropenic fever, infection |
| Colchicine | 88 | 1.2 g orally once per day | 21 | Agranulocytosis, neuritis, diarrhea, nausea/vomiting |
| Interferon α | 89-92 | Various | 0-36 | Neutropenia, fever, influenza-like symptoms, hepatotoxicity |
| Plasma exchange | 93-95 | One plasma volume exchange once per day × 1 to 8 days | 29-80 | Hypocalcemia, anaphylactoid reactions |
| Protein A immunoadsorption | 96 | Average of 6 treatments (0.25 to 2.0 L plasma per treatment) over 2 to 3 weeks | 21 | Hypersensitivity reactions, pain, nausea/vomiting |
| Cardiopulmonary complications | ||||
| Vitamin C | 97-100 | 2 g orally once per day | 0-82 | Dsypepsia, nausea/vomiting |
ATRA, all-trans retinoic acid; HSCT, hematopoietic stem cell transplant
Conclusion
Refractory ITP is challenging to treat and may be associated with poor outcomes.11,12 Our approach to management involves confirmation of the diagnosis, consideration of observation, and a tiered treatment strategy for those who require therapy (Figure 1). With recent advances in treatment, including rituximab and the TRAs, a greater proportion of patients than ever before are able to maintain hemostatic platelet counts with acceptable tolerability and safety. Still, current therapeutic options fail for a subset of highly refractory patients. For these individuals, new treatments are sorely needed. Novel agents, including an anti-CD40 ligand (BMS-986004) and splenic tyrosine kinase inhibitor (fostamatinib) as well as a number of repurposed drugs (eg, decitabine, oseltamivir, sirolimus, and thalidomide), are currently in clinical trials. We encourage patients and physicians to participate in clinical trials to support development of new treatments so that the number of patients with truly intractable disease may continue to decrease.
Authorship
Contribution: A.C. and C.E.N. searched the literature and wrote the manuscript.
Conflict-of-interest disclosure: A.C. has served as a consultant for Amgen, Bracco, CSL Behring, and Genzyme; has received research support from Spark Therapeutics and T2 Biosystems; and has provided expert witness testimony related to immune thrombocytopenia. C.E.N. has served as a consultant for Genzyme.
Correspondence: Adam Cuker, Hospital of the University of Pennsylvania, Penn Blood Disorders Center, Dulles 3, 3400 Spruce St, Philadelphia, PA 19014; e-mail: adam.cuker@uphs.upenn.edu.