Key Points
PD-L1 expression in neoplastic cells or stromal cells is associated with poor or good prognosis in ATLL, respectively.
Distinction of expression pattern of PD-L1 might be important on the point of prognostic and therapeutic markers in ATLL.
Abstract
Programmed cell death ligand 1 (PD-L1) is expressed on both tumor and tumor-infiltrating nonmalignant cells in lymphoid malignancies. The programmed cell death 1 (PD-1)/PD-L1 pathway suppresses host antitumor responses, although little is known about the significance of PD-1/PD-L1 expression in the tumor microenvironment. To investigate the clinicopathological impact of PD-L1 expression in adult T-cell leukemia/lymphoma (ATLL), we performed PD-L1 immunostaining in 135 ATLL biopsy samples. We observed 2 main groups: 1 had clear PD-L1 expression in lymphoma cells (nPD-L1(+), 7.4% of patients), and the other showed minimal expression in lymphoma cells (nPD-L1(−), 92.6%). Within the nPD-L1(−) group, 2 subsets emerged: the first displayed abundant PD-L1 expression in nonmalignant stromal cells of the tumor microenvironment (miPD-L1(+), 58.5%) and the second group did not express PD-L1 in any cell (PD-L1(−), 34.1%). nPD-L1(+) ATLL (median survival time [MST] 7.5 months, 95% CI [0.4-22.3]) had inferior overall survival (OS) compared with nPD-L1(−) ATLL (MST 14.5 months, 95% CI [10.1-20.0]) (P = .0085). Among nPD-L1(−) ATLL, miPD-L1(+) ATLL (MST 18.6 months, 95% CI [11.0-38.5]) showed superior OS compared with PD-L1(−) ATLL (MST 10.2 months, 95% CI [8.0-14.7]) (P = .0029). The expression of nPD-L1 and miPD-L1 maintained prognostic value for OS in multivariate analysis (P = .0322 and P = .0014, respectively). This is the first report describing the clinicopathological features and outcomes of PD-L1 expression in ATLL. More detailed studies will disclose clinical and biological significance of PD-L1 expression in ATLL.
Introduction
Adult T-cell leukemia/lymphoma (ATLL) is defined as a peripheral T-cell neoplasm caused by human T-cell leukemia virus type 1 (HTLV-1).1 Of the 4 clinical subtypes of ATLL, acute type and lymphoma type are considered to follow an aggressive course compared with chronic type and smoldering type.2,3 Especially in aggressive ATLL and chronic type with poor prognostic factors, an effective therapy has not been established. Chemotherapies, zidovudine/interferon-α (IFN-α) treatment, and allogenic hematopoietic stem cell transplantation deliver disappointing outcomes.4-11 Thus, new treatment strategies associated with molecular oncogenic mechanisms are being explored.
Programmed cell death ligand 1 (PD-L1) is an immunomodulatory glycoprotein expressed on antigen-presenting cells.12 Binding of PD-L1 to the programmed cell death 1 (PD-1) receptor, which is present on the surface of T cells, plays a significant role in the regulation of effector T-cell responses by suppressing cytokine production, T-cell proliferation, and T-cell adhesion.13 In various types of solid tumors, PD-L1 expression on tumor cells has been reported to associate with tumor progression and poor prognosis14-20 through inhibition of local antitumor T-cell responses.14,15 Blockade of the PD-1/PD-L1 pathway by anti-PD-1 or anti-PD-L1 monoclonal antibodies may make antitumor responses effective.21-23
In several types of lymphoid malignancies, PD-L1 is expressed on tumor cells and tumor-infiltrating nonmalignant cells, including macrophages.23,24 The presence of high levels of plasma-soluble PD-L1 and PD-L1 expression on lymphoma cells is associated with poor overall survival (OS) and is considered an important biomarker in diffuse large B-cell lymphoma (DLBCL).25,26 Moreover, blockade therapy of the PD-1/PD-L1 pathway showed a remarkable effect for Hodgkin lymphoma, in which PD-L1 is expressed on neoplastic cells.27 These results suggest that the PD-1/PD-L1 pathway might support tumor cell survival and that blockade of this pathway could be an effective therapeutic method in lymphoid malignancies other than DLBCL and Hodgkin lymphoma. Although a previous study reported that some proportion of ATLL patients showed PD-L1 expression,28 a detailed study of the clinicopathological characteristics of PD-L1 expression in ATLL is required to determine the effectiveness of anti-PD-1/PD-L1 therapies. In this study, we retrospectively investigated PD-L1 expression in ATLL and then evaluated the clinical significance between expression and clinicopathological features.
Patients, materials, and methods
Patients and samples
We reviewed 135 biopsy samples from patients newly diagnosed as ATLL. Sixty-two patients were from the international Peripheral T-cell Lymphoma Project29 and 73 patients were admitted to the Department of Pathology, Kurume University, Japan from 2006 to 2012. A tissue microarray (TMA) was created, which contained each of the 135 samples in a formalin-fixed paraffin block, where each sample was placed in a 2-mm-diameter space. The same TMA specimen slides were used in the authors’ previous study.30 Some of the morphological and immunohistochemical results in this study are consistent with the previous study.30 This study includes patients diagnosed by biopsy sample and does not include patients diagnosed by peripheral blood sample.
Cases were reclassified according to the World Health Organization classification1 by experienced hematopathologists (H.M. and K.O.). The use of patient materials and clinical information was approved by the Research Ethics Committee of Kurume University and was in accordance with the Declaration of Helsinki.
Immunohistochemical staining other than PD-L1
The antibodies used for immunohistochemistry (IHC) were anti-CD3 (Clone F7.2.38; Dako Japan), anti-CD30 (Clone Ber-H2; Dako Japan), anti-CCR4 (POTELIGEO TEST; Kyowa Medex, Tokyo, Japan), anti-FoxP3 (Clone SP97; Abcam, Tokyo, Japan), and anti-PD-1 (Clone NAT105; Abcam). In IHC analyses, except for PD-L1, samples were considered positive if more than ∼20% of the neoplastic cells were stained.
Classification of ATLL according to PD-L1 expression
Immunohistochemical staining of PD-L1 was carried out by using 2.5-μm-thick, formalin-fixed, paraffin-embedded tissue sections from the TMA for all cases. The slides were deparaffinized with xylene, followed by ethanol. After rehydration with water, antigen retrieval was performed with EDTA buffer (pH 8.0) in a microwave oven at 95°C for 20 minutes. After cooling and rinsing with buffer, the slides were placed in a Dako autostainer (Dakocytomation, Kyoto, Japan). Endogenous peroxidase activity was blocked by incubating in 3% hydrogen peroxide for 5 minutes. Slides were incubated with anti-PD-L1 rabbit monoclonal antibodies (EPR1161(2); 1:200 dilution; ab174838, Abcam) overnight at 4°C. The slides were incubated with an EnVision+ System horseradish peroxide–labeled anti-rabbit polymer (K4003; Dakocytomation) for 30 minutes. Visualization of PD-L1 was performed using diaminobenzidine for 10 minutes. Slides were counterstained with hematoxylin, dehydrated with ethanol, and mounted under coverslips.
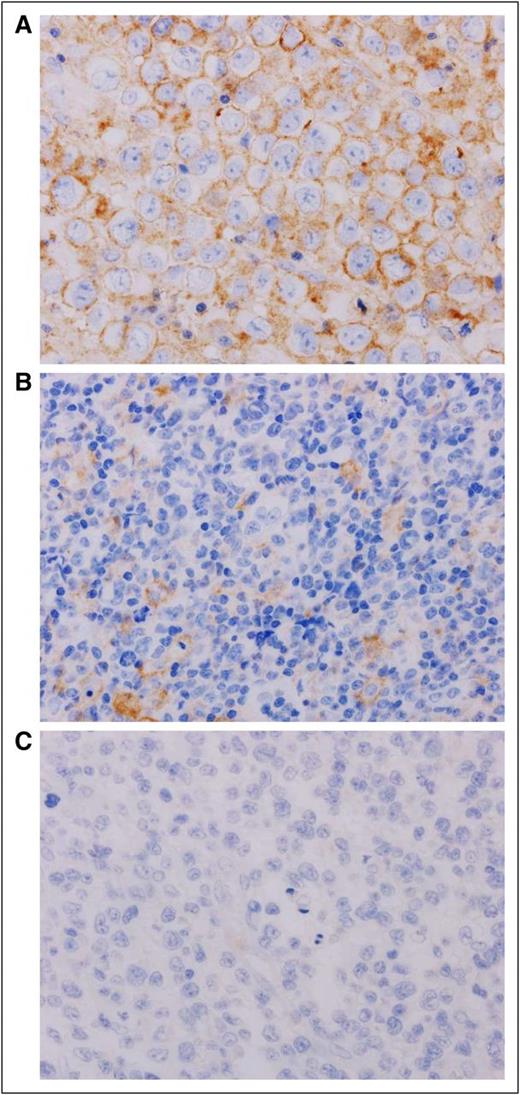
ATLL patients were categorized according to the patterns of PD-L1 expression by 2 experienced hematopathologists (H.M. and K.O.) as previously reported.25 The groups are defined as follows: (1) neoplastic PD-L1 (nPD-L1)-positive ATLL: patients with 50% or more of the lymphoma cells presenting membranous staining of PD-L1; (2) nPD-L1-negative ATLL: patients that had less than 50% PD-L1 staining of lymphoma cells; (3) microenvironmental PD-L1 (miPD-L1) -positive ATLL: a subpopulation of nPD-L1-negative ATLL patients where 10 or more PD-L1-positive nonmalignant stromal cells were observed per high power field (HPF); (4) PD-L1-negative ATLL: patients without PD-L1 expression in neoplastic cells or stromal cells (Figure 1; supplemental Figure 1, available on the Blood Web site).
Representative immunohistochemical analysis of PD-L1 expression in ATLL. (A) nPD-L1-positive ATLL. The plasma membranes of neoplastic cells were positive for PD-L1 (brown staining). (B) miPD-L1-positive ATLL. Neoplastic cells were negative for PD-L1. PD-L1-positive nonmalignant stromal cells were observed. Those stromal cells with PD-L1 expression were morphologically consistent with monocyte-derived cells, including macrophage or dendritic cells. (C) PD-L1-negative ATLL. PD-L1 was negative for both neoplastic cells and nonmalignant stromal cells. Original magnification ×600 for all panels.
Representative immunohistochemical analysis of PD-L1 expression in ATLL. (A) nPD-L1-positive ATLL. The plasma membranes of neoplastic cells were positive for PD-L1 (brown staining). (B) miPD-L1-positive ATLL. Neoplastic cells were negative for PD-L1. PD-L1-positive nonmalignant stromal cells were observed. Those stromal cells with PD-L1 expression were morphologically consistent with monocyte-derived cells, including macrophage or dendritic cells. (C) PD-L1-negative ATLL. PD-L1 was negative for both neoplastic cells and nonmalignant stromal cells. Original magnification ×600 for all panels.
Quantitative analysis of PD-1-positive TILs
We performed immunohistochemical staining for detection of PD-1 (Clone NAT105; Abcam) and quantitative analysis of PD-1-positive tumor-infiltrating lymphocytes (TILs). For evaluation of PD-1-positive TILs in each patient, up to 5 representative HPFs were selected from the specimen. The total number of PD-1-positive TILs was counted in the representative fields, and the average PD-1-positive TILs per HPF was calculated for each patient.
Statistical analysis
Clinicopathological characteristics of the patients were compared by using the χ2 test or Fisher’s 2-sided exact test. Wilcoxon rank sum test was performed when comparing PD-1-positive TIL counts between different groups. OS was defined as the time from the day of diagnosis to the day of death or the date of last follow-up. The Kaplan-Meier method was used to estimate the OS distributions, and the log-rank test was performed to determine significant differences. Univariate and multivariate Cox proportional regression models were used to evaluate the proposed prognostic factors. P < .05 were considered statistically significant. JMP version 11.0 was used in all analyses.
Results
Clinicopathological characteristics of ATLL patients
Table 1 summarizes the characteristics of the 135 patients. Median age was 67 years old with a range from 35 to 90. Seventy-six men and 59 women were included. Follow-up period ranged from 0.03 to 114.8 months with a median value of 10.9 months. With regard to Shimoyama classification, 1.8% (2/111) of the patients were categorized as smoldering type; 50.5% (56/111) were categorized as acute type, and 47.7% (53/111) were categorized as lymphoma type. A high score of the Japan Clinical Oncology Group prognostic index (JCOG-PI) was observed in 39.4% (52/132) of the patients. Regarding PD-L1 expression, 7.4% (10/135) of patients were categorized as nPD-L1 positive and 92.6% of the patients (125/135) were nPD-L1 negative. Among nPD-L1-negative patients, 58.5% (79/135) of the patients were miPD-L1 positive, and 34.1% (46/135) of the patients were PD-L1 negative. The average value of PD-1-positive TILs was 2.4 counts/HPF ranging from 0 to 38.4.
Characteristics of ATLL
| Clinical characteristic . | No. (n = 135) . | % . |
|---|---|---|
| Age, y, median (range) | 67 (35-90) | — |
| Sex, male/female | 76/59 | 56/44 |
| ECOG PS, 2-4 | 43/132 | 33 |
| Shimoyama classification | ||
| Smoldering type | 2/111 | 2 |
| Acute type | 56/111 | 51 |
| Lymphoma type | 53/111 | 48 |
| B symptom | 42/130 | 32 |
| Elevated LDH | 77 | 57 |
| Hypercalcemia | 21 | 16 |
| Elevated CRP | 80/132 | 61 |
| Ann Arbor stage, III or IV | 113/134 | 84 |
| Peripheral blood involvement | 70/132 | 53 |
| Bone marrow involvement | 30/106 | 28 |
| Skin lesion | 23/134 | 17 |
| WBC, ×109/L, median (range) | 6.6 (1.2-97.1) | — |
| Hemoglobin, g/dL, median (range) | 12.9 (6.3-18.6) | — |
| Platelets, ×109/L, median (range) | 213 (19-840) | — |
| IPI, high-intermediate or more | 78/129 | 61 |
| JCOG-PI, high | 52/132 | 39 |
| Treatment | ||
| Chemotherapy | 117/133 | 88 |
| Radiation | 14/131 | 11 |
| Transplantation | 19/131 | 15 |
| No chemotherapy or radiotherapy | 13/133 | 10 |
| CR or CR(u) | 35/116 | 30 |
| Clinical characteristic . | No. (n = 135) . | % . |
|---|---|---|
| Age, y, median (range) | 67 (35-90) | — |
| Sex, male/female | 76/59 | 56/44 |
| ECOG PS, 2-4 | 43/132 | 33 |
| Shimoyama classification | ||
| Smoldering type | 2/111 | 2 |
| Acute type | 56/111 | 51 |
| Lymphoma type | 53/111 | 48 |
| B symptom | 42/130 | 32 |
| Elevated LDH | 77 | 57 |
| Hypercalcemia | 21 | 16 |
| Elevated CRP | 80/132 | 61 |
| Ann Arbor stage, III or IV | 113/134 | 84 |
| Peripheral blood involvement | 70/132 | 53 |
| Bone marrow involvement | 30/106 | 28 |
| Skin lesion | 23/134 | 17 |
| WBC, ×109/L, median (range) | 6.6 (1.2-97.1) | — |
| Hemoglobin, g/dL, median (range) | 12.9 (6.3-18.6) | — |
| Platelets, ×109/L, median (range) | 213 (19-840) | — |
| IPI, high-intermediate or more | 78/129 | 61 |
| JCOG-PI, high | 52/132 | 39 |
| Treatment | ||
| Chemotherapy | 117/133 | 88 |
| Radiation | 14/131 | 11 |
| Transplantation | 19/131 | 15 |
| No chemotherapy or radiotherapy | 13/133 | 10 |
| CR or CR(u) | 35/116 | 30 |
| Pathological characteristics . | No. (n = 135) . | % . |
|---|---|---|
| Morphological classification | ||
| Small cell variant | 3 | 2 |
| Medium cell variant | 28 | 21 |
| Large cell variant | 57 | 42 |
| Hodgkin like variant | 2 | 2 |
| Anaplastic variant | 9 | 7 |
| Pleomorphic variant | 36 | 27 |
| IHC | ||
| nPD-L1 positive | 10 | 7 |
| nPD-L1 negative | 125 | 93 |
| mPD-L1 positive | 79 | 59 |
| PD-L1 positive | 46 | 34 |
| Neoplastic cells | ||
| PD-1, positive | 25 | 19 |
| CD30, positive | 36 | 27 |
| FoxP3, positive | 35 | 26 |
| CCR4, positive | 126 | 93 |
| PD-1-positive TIL, counts/HPF, average (range) | 2.4 (0-38.4) | — |
| Pathological characteristics . | No. (n = 135) . | % . |
|---|---|---|
| Morphological classification | ||
| Small cell variant | 3 | 2 |
| Medium cell variant | 28 | 21 |
| Large cell variant | 57 | 42 |
| Hodgkin like variant | 2 | 2 |
| Anaplastic variant | 9 | 7 |
| Pleomorphic variant | 36 | 27 |
| IHC | ||
| nPD-L1 positive | 10 | 7 |
| nPD-L1 negative | 125 | 93 |
| mPD-L1 positive | 79 | 59 |
| PD-L1 positive | 46 | 34 |
| Neoplastic cells | ||
| PD-1, positive | 25 | 19 |
| CD30, positive | 36 | 27 |
| FoxP3, positive | 35 | 26 |
| CCR4, positive | 126 | 93 |
| PD-1-positive TIL, counts/HPF, average (range) | 2.4 (0-38.4) | — |
CRP, C-reactive protein; CR, complete response/remission; CR(u), uncertain complete response/remission; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, international prognostic index; LDH, lactate dehydrogenase; WBC, white blood cell.
Clinicopathological comparison between nPD-L1-positive and -negative ATLL
Table 2 shows statistical comparisons between PD-L1 expression patterns for different clinical parameters of ATLL patients. As for Shimoyama classification, 70% of acute-type patients (7/10) and 30% of lymphoma-type patients (3/10) were nPD-L1 positive. Among nPD-L1-negative patients, 2.0% (2/101) were categorized as smoldering-type ATLL; 45.5% (46/101) were acute type ATLL, and 52.5% (53/101) were lymphoma-type ATLL. Statistical significance was not observed between nPD-L1-positive and these subtypes (P = .39). Even when smoldering type was removed from the statistical analysis, statistical significance was not achieved (P = .20). Of the miPD-L1-positive patients, 3.0% (2/67) were smoldering type, 44.8% (30/67) were acute type, and 52.2% (35/67) were lymphoma type. Among PD-L1-negative patients, 47.1% (16/34) were classified as acute type and 52.9% of patients (18/34) were lymphoma type. There was no statistical significance between miPD-L1-positive and these Shimoyama classifications (P = .43). Even in statistical analysis where smoldering type was removed, significance was not observed (P = .93).
Clinicopathological comparison among PD-L1 expression patterns in ATLL
| . | . | . | nPD-L1 negative (n = 125) . | P . | ||||
|---|---|---|---|---|---|---|---|---|
| . | nPD-L1 positive (n = 10) . | miPD-L1 positive (n = 79) . | PD-L1 negative (n = 46) . | nPD-L1+ vs nPD-L1− . | miPD-L1 + vs PD-L1− . | |||
| Characteristic . | No. . | % . | No. . | % . | No. . | % . | P* . | P† . |
| Sex (male/female) | 7/3 | 70/30 | 40/39 | 51/49 | 29/17 | 63/73 | .51‡ | .18 |
| Age, y | ||||||||
| Median (range) | 66 (47–82) | 66 (35–90) | 64 (40–86) | .78 | .34 | |||
| ECOG PS, 2-4 | 5 | 50 | 26/77 | 34 | 12/45 | 27 | .29‡ | .41 |
| Shimoyama classification | .39 | .43 | ||||||
| Smoldering type | 0 | 0 | 2/67 | 3 | 0/34 | 0 | ||
| Acute type | 7 | 70 | 30/67 | 45 | 16/34 | 47 | [.20‡] | [.93] |
| Lymphoma type | 3 | 30 | 35/67 | 52 | 18/34 | 53 | ||
| B symptoms | 5 | 50 | 26/74 | 35 | 11 | 24 | .29‡ | .91 |
| Elevated serum LDH | 6 | 60 | 43 | 54 | 28 | 61 | 1.00‡ | .48 |
| Hypercalcemia | 2 | 20 | 8 | 10 | 11 | 24 | .65‡ | .04 |
| Elevated CRP | 8 | 80 | 44/76 | 58 | 28 | 61 | .31‡ | .75 |
| Ann Arbor stage, III or IV | 10 | 100 | 64/78 | 82 | 39 | 85 | .36‡ | .69 |
| Peripheral blood involvement | 6 | 60 | 41/76 | 54 | 23 | 50 | .75‡ | .67 |
| Bone marrow involvement | 4/7 | 57 | 19/65 | 29 | 7/34 | 21 | .098‡ | .35 |
| Skin lesion | 1 | 10 | 19/78 | 24 | 3 | 7 | 1.00‡ | .01‡ |
| WBC, ×109/L, median (range) | 14.8 (2.9-48.3) | 8.6 (1.2-97.1) | 9.4 (1.6-45.9) | .17 | .55 | |||
| Hemoglobin, g/dL, median (range) | 12.0 (7.7-14.7) | 12.4 (6.3-16.1) | 13.2 (8.0-18.6) | .49 | .052 | |||
| Platelets, ×109/L, median (range) | 193 (65-370) | 222 (19-538) | 240 (22-840) | .24 | .46 | |||
| IPI risk group, high (3-5) | 9 | 90 | 42/74 | 57 | 27/45 | 60 | .09* | .73 |
| JCOG-PI, high | 5/10 | 50 | 28/77 | 36 | 19/45 | 42 | .51 | .52 |
| Treatment | ||||||||
| Chemotherapy | 8 | 80 | 69/78 | 89 | 40/45 | 89 | .34‡ | .94 |
| Radiotherapy | 0 | 0 | 9/77 | 12 | 5/44 | 11 | .60‡ | 1.00 |
| Transplantation | 1 | 10 | 10/77 | 13 | 8/44 | 18 | 1.00‡ | .45 |
| No therapy | 2 | 20 | 9/78 | 12 | 2/45 | 4 | .25‡ | .33 |
| CR or CR(u) | 2/8 | 25 | 22/67 | 33 | 11/41 | 27 | 1.00‡ | .51 |
| Morphological classification | .51 | .54 | ||||||
| Small-cell variant | 0 | 0 | 2 | 3 | 1 | 2 | ||
| Medium-cell variant | 2 | 20 | 19 | 24 | 7 | 15 | ||
| Large-cell variant | 2 | 20 | 31 | 39 | 24 | 52 | ||
| Hodgkin-like variant | 0 | 0 | 2 | 3 | 0 | 0 | ||
| Anaplastic variant | 1 | 10 | 5 | 6 | 3 | 7 | ||
| Pleomorphic variant | 5 | 50 | 20 | 25 | 11 | 24 | ||
| IHC | ||||||||
| Neoplastic cells | ||||||||
| PD-1, positive | 0 | 0 | 16 | 20 | 9 | 20 | .21‡ | .93 |
| CD30, positive | 5 | 50 | 20 | 25 | 11 | 24 | .13‡ | .86 |
| CCR4, positive | 10 | 100 | 72 | 91 | 44 | 96 | 1.00‡ | .48‡ |
| FoxP3, positive | 2 | 20 | 21 | 27 | 12 | 26 | 1.00‡ | .95 |
| PD-1 positive TIL, counts/HPF, average (range) | 3.0 (0-24.4) | 3.6 (0-38.4) | 0.3 (0-5.2) | .16 | .0009 | |||
| . | . | . | nPD-L1 negative (n = 125) . | P . | ||||
|---|---|---|---|---|---|---|---|---|
| . | nPD-L1 positive (n = 10) . | miPD-L1 positive (n = 79) . | PD-L1 negative (n = 46) . | nPD-L1+ vs nPD-L1− . | miPD-L1 + vs PD-L1− . | |||
| Characteristic . | No. . | % . | No. . | % . | No. . | % . | P* . | P† . |
| Sex (male/female) | 7/3 | 70/30 | 40/39 | 51/49 | 29/17 | 63/73 | .51‡ | .18 |
| Age, y | ||||||||
| Median (range) | 66 (47–82) | 66 (35–90) | 64 (40–86) | .78 | .34 | |||
| ECOG PS, 2-4 | 5 | 50 | 26/77 | 34 | 12/45 | 27 | .29‡ | .41 |
| Shimoyama classification | .39 | .43 | ||||||
| Smoldering type | 0 | 0 | 2/67 | 3 | 0/34 | 0 | ||
| Acute type | 7 | 70 | 30/67 | 45 | 16/34 | 47 | [.20‡] | [.93] |
| Lymphoma type | 3 | 30 | 35/67 | 52 | 18/34 | 53 | ||
| B symptoms | 5 | 50 | 26/74 | 35 | 11 | 24 | .29‡ | .91 |
| Elevated serum LDH | 6 | 60 | 43 | 54 | 28 | 61 | 1.00‡ | .48 |
| Hypercalcemia | 2 | 20 | 8 | 10 | 11 | 24 | .65‡ | .04 |
| Elevated CRP | 8 | 80 | 44/76 | 58 | 28 | 61 | .31‡ | .75 |
| Ann Arbor stage, III or IV | 10 | 100 | 64/78 | 82 | 39 | 85 | .36‡ | .69 |
| Peripheral blood involvement | 6 | 60 | 41/76 | 54 | 23 | 50 | .75‡ | .67 |
| Bone marrow involvement | 4/7 | 57 | 19/65 | 29 | 7/34 | 21 | .098‡ | .35 |
| Skin lesion | 1 | 10 | 19/78 | 24 | 3 | 7 | 1.00‡ | .01‡ |
| WBC, ×109/L, median (range) | 14.8 (2.9-48.3) | 8.6 (1.2-97.1) | 9.4 (1.6-45.9) | .17 | .55 | |||
| Hemoglobin, g/dL, median (range) | 12.0 (7.7-14.7) | 12.4 (6.3-16.1) | 13.2 (8.0-18.6) | .49 | .052 | |||
| Platelets, ×109/L, median (range) | 193 (65-370) | 222 (19-538) | 240 (22-840) | .24 | .46 | |||
| IPI risk group, high (3-5) | 9 | 90 | 42/74 | 57 | 27/45 | 60 | .09* | .73 |
| JCOG-PI, high | 5/10 | 50 | 28/77 | 36 | 19/45 | 42 | .51 | .52 |
| Treatment | ||||||||
| Chemotherapy | 8 | 80 | 69/78 | 89 | 40/45 | 89 | .34‡ | .94 |
| Radiotherapy | 0 | 0 | 9/77 | 12 | 5/44 | 11 | .60‡ | 1.00 |
| Transplantation | 1 | 10 | 10/77 | 13 | 8/44 | 18 | 1.00‡ | .45 |
| No therapy | 2 | 20 | 9/78 | 12 | 2/45 | 4 | .25‡ | .33 |
| CR or CR(u) | 2/8 | 25 | 22/67 | 33 | 11/41 | 27 | 1.00‡ | .51 |
| Morphological classification | .51 | .54 | ||||||
| Small-cell variant | 0 | 0 | 2 | 3 | 1 | 2 | ||
| Medium-cell variant | 2 | 20 | 19 | 24 | 7 | 15 | ||
| Large-cell variant | 2 | 20 | 31 | 39 | 24 | 52 | ||
| Hodgkin-like variant | 0 | 0 | 2 | 3 | 0 | 0 | ||
| Anaplastic variant | 1 | 10 | 5 | 6 | 3 | 7 | ||
| Pleomorphic variant | 5 | 50 | 20 | 25 | 11 | 24 | ||
| IHC | ||||||||
| Neoplastic cells | ||||||||
| PD-1, positive | 0 | 0 | 16 | 20 | 9 | 20 | .21‡ | .93 |
| CD30, positive | 5 | 50 | 20 | 25 | 11 | 24 | .13‡ | .86 |
| CCR4, positive | 10 | 100 | 72 | 91 | 44 | 96 | 1.00‡ | .48‡ |
| FoxP3, positive | 2 | 20 | 21 | 27 | 12 | 26 | 1.00‡ | .95 |
| PD-1 positive TIL, counts/HPF, average (range) | 3.0 (0-24.4) | 3.6 (0-38.4) | 0.3 (0-5.2) | .16 | .0009 | |||
Abbreviations are explained in Table 1.
nPD-L1 positive versus nPD-L1 negative.
miPD-L1 positive versus PD-L1 negative.
Fisher’s exact test; values in brackets indicate P value between acute type and lymphoma type.
Clinicopathological comparison between miPD-L1-positive and PD-L1-negative ATLL
Compared with the PD-L1-negative group, the miPD-L1-positive group was significantly associated with a low proportion of hypercalcemia (P = .042) and a high proportion of skin lesions (P = .014).
Quantitative analysis of PD-1-positive TILs
In regards to PD-1-positive TILs, the nPD-L1-positive group showed an average of 3.0 counts/HPF (median 0.3; range 0-24.4) and the nPD-L1-negative group averaged 2.3 counts/HPF (median 0; range 0-38.4). Among nPD-L1-negative patients, the miPD-L1-positive group averaged 3.6 counts/HPF (median 0; range, 0-38.4) and the PD-L1-negative group had 0.3 counts/HPF (median 0; range 0-5.2). The nPD-L1-positive group did not show a significant difference in PD-1-positive TILs compared with the nPD-L1-negative group (P = .162); however, there was a significantly higher number of PD-1-positive TILs in the nPD-L1-positive group compared with the PD-L1-negative group (P = .0041). miPD-L1-positive patients had significantly more PD-1-positive TILs than the PD-L1-negative group (P = .0009).
OS in ATLL patients according to PD-L1 expression
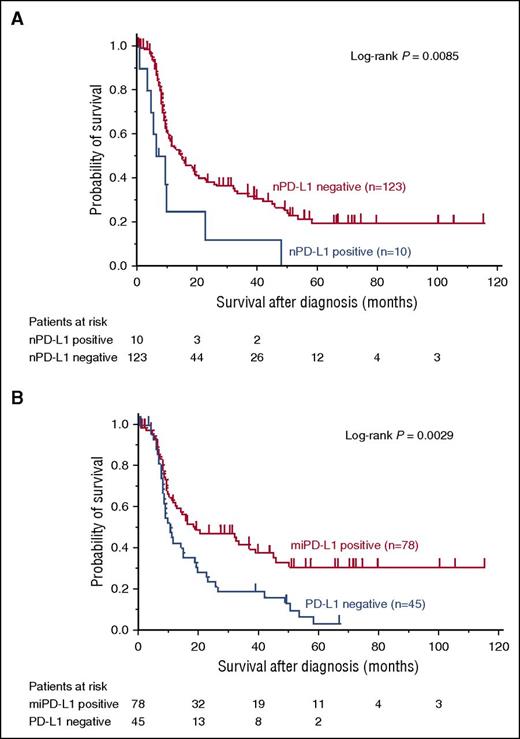
nPD-L1-positive patients (median survival time [MST] 7.5 months, 95% confidence interval [CI] [0.4-22.3]) had inferior OS compared with nPD-L1-negative patients (MST 14.5 months, 95% CI [10.1-20.0]) (P = .0085; Figure 2A). Among nPD-L1-negative patients, miPD-L1-positive patients (MST 18.6 months, 95% CI [11.0-38.5]) showed superior OS than PD-L1-negative patients (MST 10.2 months, 95% CI [8.0-14.7]) (P = .0029; Figure 2B).
OS of ATLL among the 4 PD-L1 expression groups. (A) nPD-L1 positive ATLL shows worse OS compared with nPD-L1 negative ATLL (P = .0085). (B) miPD-L1 positive ATLL shows better OS compared with PD-L1 negative ATLL (P = .0029).
OS of ATLL among the 4 PD-L1 expression groups. (A) nPD-L1 positive ATLL shows worse OS compared with nPD-L1 negative ATLL (P = .0085). (B) miPD-L1 positive ATLL shows better OS compared with PD-L1 negative ATLL (P = .0029).
Univariate analyses identified the following variables as prognostic factors: nPD-L1 expression (hazard ratio [HR], 2.455; 95% CI, 1.144-4.642; P = .0234), miPD-L1 expression (HR, 0.525; 95% CI, 0.341-0.811; P = .0039), high JCOG-PI (HR, 1.777; 95% CI, 1.149-2.710; P = .0103), and age over 70 (HR, 1.647; 95% CI, 1.060-2.515; P = .0269) (Table 3). Multivariate analysis with nPD-L1 expression and other factors showed that nPD-L1 expression remained a significant prognostic factor (HR, 2.373; 95% CI, 1.084-4.625; P = .0322) (Table 3, Multivariate analysis 1). A similar multivariate analysis with miPD-L1 expression indicated that miPD-L1 expression persisted as a significant prognostic factor (HR, 0.481; 95% CI, 0.309-0.750; P = .0014) (Table 3, Multivariate analysis 2).
Prognostic factors affecting the OS of patients with ATLL
| Characteristics . | Univariate analysis . | Multivariate analysis 1* . | Multivariate analysis 2† . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| nPD-L1, positive (vs nPD-L1 negative) | 2.455 (1.144-4.642) | .0234 | 2.373 (1.084-4.625) | .0322 | ||
| miPD-L1, positive (vs PD-L1 negative) | 0.525 (0.341-0.811) | .0039 | 0.48 1(0.309-0.750) | .0014 | ||
| JCOG-PI, high (vs low) | 1.777 (1.149-2.710) | .0103 | 1.750 (1.114-2.721) | .0157 | 1.797 (1.112-2.863) | .0172 |
| Age, >70 (vs ≤70) | 1.647 (1.060-2.515) | .0269 | 1.694 (1.080-2.612) | .0224 | 1.656 (1.027-2.615) | .0389 |
| Ann Arbor stage, III/IV (vs I/II) | 1.776 (0.988-3.530) | .0551 | 1.640 (0.903-3.283) | .1081 | 1.829 (0.999-3.687) | .0503 |
| LDH, elevated (vs normal) | 1.000 (0.664-1.513) | .9986 | 0.850 (0.554-1.309) | .4575 | 0.945 (0.599-1.671) | .8089 |
| Characteristics . | Univariate analysis . | Multivariate analysis 1* . | Multivariate analysis 2† . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| nPD-L1, positive (vs nPD-L1 negative) | 2.455 (1.144-4.642) | .0234 | 2.373 (1.084-4.625) | .0322 | ||
| miPD-L1, positive (vs PD-L1 negative) | 0.525 (0.341-0.811) | .0039 | 0.48 1(0.309-0.750) | .0014 | ||
| JCOG-PI, high (vs low) | 1.777 (1.149-2.710) | .0103 | 1.750 (1.114-2.721) | .0157 | 1.797 (1.112-2.863) | .0172 |
| Age, >70 (vs ≤70) | 1.647 (1.060-2.515) | .0269 | 1.694 (1.080-2.612) | .0224 | 1.656 (1.027-2.615) | .0389 |
| Ann Arbor stage, III/IV (vs I/II) | 1.776 (0.988-3.530) | .0551 | 1.640 (0.903-3.283) | .1081 | 1.829 (0.999-3.687) | .0503 |
| LDH, elevated (vs normal) | 1.000 (0.664-1.513) | .9986 | 0.850 (0.554-1.309) | .4575 | 0.945 (0.599-1.671) | .8089 |
The variables included in multivariate analysis 1 for OS were nPD-L1, JCOG-PI, age, Ann Arbor stage, and LDH.
The variables included in multivariate analysis 2 for OS were miPD-L1, JCOG-PI, age, Ann Arbor stage, and LDH.
Discussion
In the present study, we demonstrated for the first time that PD-L1 expression on ATLL tumor cells is an independent poor prognostic factor for OS and that PD-L1 expression on nonmalignant stromal cells was an independent good prognostic factor for OS in ATLL.
Many previous reports have showed that high PD-L1 expression in tumor cells is associated with clinical progression and worse OS in other neoplasms, including DLBCL.8,25,31 Although PD-L1-expressing cells have various mechanisms to escape T-cell immunity,15 the bad prognoses associated with PD-L1 expression in neoplastic cells is thought to be caused by the activity of the PD-1/PD-L1 pathway. This pathway induces T-cell-mediated immune suppression in the tumor microenvironment.14 Some in vitro studies demonstrated that the binding of PD-L1 in neoplastic cells to PD-1 on T cells negatively regulates synthesis of interleukin-2 and IFN-γ. Suppressed secretion of interleukin-2 and IFN-γ from PD-1-positive T cells consequently leads to apoptosis of CD8+ cytotoxic T lymphocytes.14,31 DLBCL cell lines with PD-L1 expression also exhibited inhibition of T-cell proliferation and IFN-γ secretion by tumor-associated T cells.23 Although these results are not enough to explain the precise underlying mechanisms, they do suggest that the PD-1/PD-L1 pathway may contribute to a worse clinical outcome in ATLL patients with PD-L1 expression on neoplastic cells.
This study found that PD-L1 expression on stromal cells in the tumor microenvironment correlated with better prognosis. Stromal cells with PD-L1 expression showed roundlike nuclei with abundant and poorly defined cytoplasms. These cells may be monocyte-derived cells, including macrophage and dendritic cells. Some of the stromal cells with PD-L1 expression were classified as macrophages based on IHC (supplemental Figure 1). Previous reports suggest that PD-L1 may be upregulated in activated macrophages and dendritic cells.12,32 As for lymphoid malignancies, macrophages in the microenvironment have been shown to exhibit PD-L1 expression in Hodgkin lymphoma and DLBCL.24,33 Some kinds of dendritic cells are also known to express PD-L1 in reactive lymphoid tissue.34 However, the biological significance of PD-L1 expression on macrophages and dendritic cells in the tumor microenvironment remains unclear. More detailed research of stromal cells with PD-L1 expression is needed to understand the biological effect in lymphoid malignancies.
Concerning the association between better prognosis and PD-L1 expression, few studies have demonstrated the association between PD-L1 expression in stromal cells and good prognosis.35,36 In contrast, PD-L1 expression in neoplastic cells is reported to be a good prognostic factor in several malignancies.35-37 In those malignancies, PD-L1 expression in neoplastic and stromal cells was associated with increased IFN-γ and TILs, including CD8-positive and regulatory T cells.35-37 An in vitro study reported that CD8-positive T cells and IFN-γ induced PD-L1 expression of stromal cells in the microenvironment.38 These results suggest the possibility that the better prognosis of miPD-L1 expression in ATLL is not the result of an upregulated PD-1/PD-L1 pathway; miPD-L1 expression may simply be an indicator of a different immune condition in which antitumor immunity is much more activated.
In our quantitative analysis of PD-1-positive TILs, the numbers of PD-1-positive TILs were higher in nPD-L1- and miPD-L1-positive patients than those in PD-L1-negative patients. In various other cancers such as melanoma and sarcoma, PD-L1 expression on neoplastic cells and increased PD-1-positive TILs cause worse prognosis.39-41 These results suggest that, in ATLL, neoplastic cells with PD-L1 expression and PD-1-positive TILs might activate the PD-L1/PD-1 pathway that suppresses the T-cell immune response against neoplastic cells. On the other hand, the significance of a high number of PD-1-positive TILs in miPD-L1-positive patients associated with better prognosis is unknown. In follicular lymphoma and DLBCL, a high number of PD-1-positive TILs are associated with better prognosis regardless of PD-L1 expression.25,42,43 Nevertheless, most malignancies with a high number of PD-1-positive TILs show worse OS probably due to activation of the PD-L1/PD-1 pathway.39-41 The association between better prognosis and high levels of PD-1-positive TILs in FL and DLBCL is presumably caused not by the characteristics of PD-1-positive T cells associated with tumor-immunity, but by those of bystander follicular helper T cells. These cells coexist with neoplastic B cells in the germinal center and might not play a specific role in tumor immunity.25,42,43 A high level of PD-1-positive TILs and PD-L1 expression in stromal cells in ATLL could similarly indicate other pathological or immune conditions. More detailed study of PD-1-positive TILs will elucidate the mechanism underlying these associations.
Some studies have shown an association with PD-L1 expression and HTLV-1 infection. A previous report25 showed that PD-L1 expression was not observed in lymphocytes of asymptomatic HTLV-1 carriers, but PD-L1 expression did occur in some ATLL cases. Furthermore, when PD-L1-positive neoplastic cells from ATLL patients were treated with anti-PD-L1 antagonistic antibody, the HTLV-1-specific CD8+ T-cell response was upregulated. These results suggest that the PD-1/PD-L1 pathway may play a significant role in maintenance of persistent HTLV-1 infection25 ; specifically, this pathway might promote immune escape of neoplastic cells and encourage progression of ATLL. In regards to PD-L1 expression on nonmalignant cells in the tumor microenvironment, we could not show the association between other markers of infection (CCR4) and PD-L1 expression in microenvironment cells. More detailed studies could possibly reveal an association.
Immunotherapy against the PD1/PD-L1 pathway is reported as an effective treatment in several malignancies.22 In DLBCL and Hodgkin lymphoma, anti-PD-1 monoclonal antibody treatment (pidilizumab, nivolumab, and so forth) achieved marked improvement of clinical outcome.27,44 This study suggests that immunotherapy targeting the PD1/PD-L1 pathway for nPD-L1-positive ATLL could result in a better clinical outcome. On the other hand, administration of immunotherapy against the PD-1/PD-L1 pathway for miPD-L1-positive ATLL should be carefully considered because stromal cells with PD-L1 expression and PD-1-positive TILs possibly could lead to better clinical outcome. In any case, careful clinical studies are needed to determine whether blockade of the PD-1/PD-L1 pathway can be an effective treatment strategy for patients of ATLL according to PD-L1 expression patterns.
There are some limitations in this study. First, the number of cases is relatively small. Regardless, statistical significance was partially observed. Further studies with larger populations are warranted to validate our results. Second, this study includes only patients diagnosed by biopsy specimen and does not contain diagnoses by peripheral blood sample. The selection of only 1 sampling method might lend bias to these findings. Further studies that include patients diagnosed by peripheral blood sample are required to confirm our results. Third, this study could not evaluate all subtypes of Shimoyama classification partially because of shortages of clinical information for some patients. More studies, including all Shimoyama classifications, are needed to examine whether these findings are true for chronic-type and smoldering-type ATLL. Fourth, PD-L1 expression in this study was evaluated only with IHC. Additional analyses for genomic abnormalities and transcriptional levels of PD-L1 will be required to understand the biological and clinical significance of PD-L1 expression. Last, it is still unknown whether PD-1/PD-L1 blockade should actually be administered to ATLL with PD-L1 expression on neoplastic cells or stromal cells. Future clinical analyses are needed to determine if PD-1/PD-L1 blockade will lead to better clinical outcomes for ATLL patients.
In conclusion, we have demonstrated that PD-L1 expression on neoplastic cells and stromal cells in ATLL induces worse or better prognosis, respectively. These results suggest that the association between clinical outcome and PD-L1 expression in neoplastic cells and stromal cells could be caused by tumor microenvironment, including antitumor immunity. The administration of PD-1/PD-L1 blockade should be selected carefully according to expression patterns of PD-L1 in ATLL because PD-L1 expression in stromal cells may be associated with better clinical outcome. More detailed studies will disclose the clinical and biological significance of PD-L1 expression in ATLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kazutaka Nakashima, Mayumi Miura, Kanoko Miyazaki, Yuki Morotomi, Chie Kuroki, and Kaoruko Nagatomo for their technical assistance.
This research was supported by a Grant-in-Aid for Scientific Research by the Ministry of Education, Culture, Sports, Science, and Technology, Japan, by Japan Society for the Promotion of Science (KAKENHI) grant number JP16K19093, and by a grant from Practical Research for Innovative Cancer Control Agency for Medical Research and Development (15ck0106015h0002) (Y.I., M.S., and K.O.). This study was performed using tissue samples obtained from the many hospitals and/or institutes included in the Kyushu Lymphoma Study Group: Department of Medicine and Biosystemic Science, Kyushu University Faculty of Medicine; Department of Hematology, Karatsu Red Cross Hospital; Department of Hematology, Sasebo City General Hospital; Department of Hematology, Atomic Bomb Disease and Hibakusha Medicine Unit, Atomic Bomb Disease Institute, Nagasaki University; and Department of Medicine and Bioregulatory Science, Graduate School of Medical Sciences, Kyushu University. The authors express their appreciation for the donated samples.
Authorship
Contribution: H.M. and K.O. were responsible for conception and design; T.K., K. Kato, H.T., and Y.I. provided study materials or patients; H.M., J.K., T.K., J.S., Y.S., D.K., and K. Kawamoto performed collection and assembly of data; H.M., J.K., T.K., N.Y., S.Y., M.S., and K.O. performed data analysis and interpretation; H.M. wrote the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hiroaki Miyoshi, Department of Pathology, School of Medicine, Kurume University, Asahimachi 67, Kurume-city, Fukuoka 830-0011, Japan; e-mail: miyoshi_hiroaki@med.kurume-u.ac.jp.