Key Points
Cord blood is a rich source of B cells with immunoregulatory function.
IL-10–producing B cells may protect against cGVHD after cord blood transplantation.
Abstract
Cord blood (CB) offers a number of advantages over other sources of hematopoietic stem cells, including a lower rate of chronic graft-versus-host disease (cGVHD) in the presence of increased HLA disparity. Recent research in experimental models of autoimmunity and in patients with autoimmune or alloimmune disorders has identified a functional group of interleukin-10 (IL-10)-producing regulatory B cells (Bregs) that negatively regulate T-cell immune responses. At present, however, there is no consensus on the phenotypic signature of Bregs, and their prevalence and functional characteristics in CB remain unclear. Here, we demonstrate that CB contains an abundance of B cells with immunoregulatory function. Bregs were identified in both the naive and transitional B-cell compartments and suppressed T-cell proliferation and effector function through IL-10 production as well as cell-to-cell contact involving CTLA-4. We further show that the suppressive capacity of CB-derived Bregs can be potentiated through CD40L signaling, suggesting that inflammatory environments may induce their function. Finally, there was robust recovery of IL-10–producing Bregs in patients after CB transplantation, to higher frequencies and absolute numbers than seen in the peripheral blood of healthy donors or in patients before transplant. The reconstituting Bregs showed strong in vitro suppressive activity against allogeneic CD4+ T cells, but were deficient in patients with cGVHD. Together, these findings identify a rich source of Bregs and suggest a protective role for CB-derived Bregs against cGVHD development in CB recipients. This advance could propel the development of Breg-based strategies to prevent or ameliorate this posttransplant complication.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative option for many patients with high-risk hematologic malignancies.1 However, ∼70% of patients who require an allograft will lack an HLA-identical sibling donor, and many in this group will lack a suitably matched unrelated donor.2 Because of the less stringent requirement for HLA matching, human cord blood (CB) is widely used as a source of hematopoietic stem cells for many patients without a suitable donor.3-5 Although the rate of acute graft-versus-host disease (GVHD) is higher after double-unit compared with single-unit transplantation (cord blood transplantation [CBT]),6,7 a lower incidence of chronic GVHD (cGVHD) has been reported after either single or double CBT than after the use of other stem cell sources, despite broader HLA disparity.3-5
Donor-derived CD4+ and CD8+ T lymphocytes are classically considered the chief effector cells arbitrating the pathogenesis of acute GVHD and cGVHD.8,9 Several independent lines of evidence clearly demonstrate a critical breakdown in peripheral B-cell tolerance and insufficient immune regulation after allogeneic HSCT.10 Indeed, B cells isolated from patients with cGVHD are typically activated with increased signaling through the AKT and extracellular signal-regulated kinase pathways.11,12 Interleukin-10 (IL-10)–producing B cells (B10 cells) are a newly described subset of B cells with regulatory function. Mizoguchi and collaborators, who identified regulatory B cells (Bregs) as an IL-10–producing B-cell subset, introduced the term “regulatory B cells.”13 Since these seminal observations, a considerable body of evidence has conclusively demonstrated the significance of IL-10–producing Bregs in diverse murine models and human studies of autoimmunity, infection, and cancer.14-20 More recently, there have also been reports of the role of Bregs in human cGVHD.18,19
To date, the limited number of cell surface antigens studied and the lack of consensual definitions of the Breg subset phenotype have impeded direct comparison of human B-cell subsets with regulatory function. In murine models, B cells with regulatory function were found within CD1dhiCD5+ (B10) cells, mesenteric lymph node B cells, marginal zone B cells, T2− marginal zone precursor cells, and Tim-1+ Bregs.17,21,22 In humans, Blair and coworkers have described Bregs as CD19+CD24hiCD38hi, a phenotype that normally defines human transitional B cells,21,22 whereas other lines of evidence indicate that human Bregs, identified through IL-10 intracellular staining, are contained within the CD24hiCD27+ B-cell subset19,23 or within both the memory (CD27+) and transitional (CD38hi) B-cell compartments.24 We recently reported that Bregs are enriched within both the transitional and immunoglobulin M (IgM) memory B-cell subsets in human peripheral blood (PB), and mediate suppression of T-cell proliferation and effector cytokine production through both IL-10–dependent and cell-cell contact-dependent mechanisms (mainly involving CD80/CD86).18 We also showed that Bregs are deficient in patients with cGVHD after HLA-matched sibling or matched unrelated donor HSCT.18
Whereas CD19+CD24hiCD38hi transitional B cells represent only about 4% of the B cells in healthy adult peripheral blood, they comprise nearly 50% of B cells in CB, with their frequency progressively declining during infancy.25,26 In contrast to PB, CD24hiCD38−CD27+ memory B cells are absent in CB and only become detectable in the first year of life.27,28 Thus, given the strikingly higher prevalence of B cells with a regulatory phenotype in CB, we hypothesized that this property may contribute to the lower rates of cGVHD after CB transplantation. Here, we show that IL-10–producing B cells with T regulatory cell (Treg)-independent immunosuppressive properties are highly enriched in both the naive and transitional B-cell compartments in CB. They suppress T cells through the production of IL-10, as well as by cell-to-cell contact-mediated mechanisms involving CTLA-4. We also demonstrate a robust recovery of IL-10–producing B cells by 6 months post-CBT, with significantly greater frequencies and absolute numbers than seen in the PB of healthy donors or in patients before CBT. Furthermore, Breg reconstitution in patients with cGVHD was significantly lower than in CBT recipients without this complication. These findings suggest a protective role for CB-derived Bregs against the development of cGVHD in CBT recipients, and support the testing of strategies to exploit Breg-based therapy in the treatment of this complication.
Material and methods
Patients and controls
All patient samples were collected after written informed consent was given in accord with local policy guidelines at the MD Anderson Cancer Center (MDACC) and the Declaration of Helsinki. Patient characteristics are summarized in Table 1.
Clinical characteristics of the patients
| . | cGVHD, n = 12 . | No cGVHD, n = 15 . | P value . |
|---|---|---|---|
| Age, median (range), y | 34 (25-66) | 43 (21-64) | .4255 |
| Sex, n (%) | |||
| Female | 6 (50) | 11 (73) | |
| Male | 6 (50) | 4 (26.7) | |
| Race, n (%) | |||
| White | 5 (41.7) | 11 (73) | |
| Black | 3 (25) | 0 (0) | |
| Hispanic | 2 (16.7) | 3 (20) | |
| Asian | 2 (16.7) | 1 (7) | |
| HLA matching, n (%) | |||
| 4/6 and 4/6 | 7 (58.3) | 8 (53.3) | |
| 4/6 and 5/6 | 1 (8.3) | 3 (20) | |
| 5/6 and 5/6 | 3 (25) | 3 (20) | |
| Conditioning, n (%) | |||
| Flu/Cy/TBI | 2 (16.7) | 4 (26.7) | |
| Flu/Mel/Thio | 7 (58.3) | 6 (40) | |
| Flu/Mel140 | 0 (0) | (13.3) | |
| Bu/Flu/Clo/TBI | 3 (25) | 3 (20) | |
| Diagnosis, n (%) | |||
| Primary AML | 6 (50) | 7 (46.7) | |
| Secondary AML | 2 (16.7) | 3 (20) | |
| CML | 1 (8.3) | 1 (7) | |
| CLL/NHL | 2 (16.7) | 3 (20) | |
| Hodgkin disease | 1 (8.3) | 1 (7) | |
| Cytogenetics, n (%) | |||
| Favorable | 3 (25) | 0 (0) | |
| Intermediate | 7 (58.3) | 6 (40) | |
| Unfavorable | 2 (16.7) | 7 (46) | |
| Disease status at transplant, n (%) | |||
| CR1 | 8 (66.7) | 8 (53.3) | |
| CR2/CR3 | 3 (20) | 4 (26.7) | |
| Active disease | 1 (8.3) | 3 (20) | |
| ALC, median (range), 103/µL | 0.84 (0.41-2.40) | 0.78 (0.05-5.28) | .44 |
| Day 30 ALC, median (range), 103/µL | 0.47 (0.14-0.73) | 0.22 (0.08-0.931) | .54 |
| . | cGVHD, n = 12 . | No cGVHD, n = 15 . | P value . |
|---|---|---|---|
| Age, median (range), y | 34 (25-66) | 43 (21-64) | .4255 |
| Sex, n (%) | |||
| Female | 6 (50) | 11 (73) | |
| Male | 6 (50) | 4 (26.7) | |
| Race, n (%) | |||
| White | 5 (41.7) | 11 (73) | |
| Black | 3 (25) | 0 (0) | |
| Hispanic | 2 (16.7) | 3 (20) | |
| Asian | 2 (16.7) | 1 (7) | |
| HLA matching, n (%) | |||
| 4/6 and 4/6 | 7 (58.3) | 8 (53.3) | |
| 4/6 and 5/6 | 1 (8.3) | 3 (20) | |
| 5/6 and 5/6 | 3 (25) | 3 (20) | |
| Conditioning, n (%) | |||
| Flu/Cy/TBI | 2 (16.7) | 4 (26.7) | |
| Flu/Mel/Thio | 7 (58.3) | 6 (40) | |
| Flu/Mel140 | 0 (0) | (13.3) | |
| Bu/Flu/Clo/TBI | 3 (25) | 3 (20) | |
| Diagnosis, n (%) | |||
| Primary AML | 6 (50) | 7 (46.7) | |
| Secondary AML | 2 (16.7) | 3 (20) | |
| CML | 1 (8.3) | 1 (7) | |
| CLL/NHL | 2 (16.7) | 3 (20) | |
| Hodgkin disease | 1 (8.3) | 1 (7) | |
| Cytogenetics, n (%) | |||
| Favorable | 3 (25) | 0 (0) | |
| Intermediate | 7 (58.3) | 6 (40) | |
| Unfavorable | 2 (16.7) | 7 (46) | |
| Disease status at transplant, n (%) | |||
| CR1 | 8 (66.7) | 8 (53.3) | |
| CR2/CR3 | 3 (20) | 4 (26.7) | |
| Active disease | 1 (8.3) | 3 (20) | |
| ALC, median (range), 103/µL | 0.84 (0.41-2.40) | 0.78 (0.05-5.28) | .44 |
| Day 30 ALC, median (range), 103/µL | 0.47 (0.14-0.73) | 0.22 (0.08-0.931) | .54 |
ALC, absolute lymphocyte count; AML, acute myeloid leukemia; Bu, busulfan; CLL, chronic lymphocytic leukemia; Clo, clofarabine; CML, chronic myeloid leukemia; CR, complete remission; Cy, cyclophosphamide; Flu, fludarabine; Mel140, melphalan 140 mg/m2; NHL, non-Hodgkin lymphoma; TBI, total body irradiation; Thio, thiotepa.
Human cell isolation
Details of human isolation protocols are included in the supplemental Material and methods (available on the Blood Web site).
Characterization of IL-10+CD19+ B cells in CB and PB from CBT recipients
IL-10+ B cells from CBT recipients were characterized by intracellular cytokine detection as previously described.18 Details of the assay are included in the supplemental Material and methods.
Proliferation and cytokine suppression
T-cell proliferation and cytokine function were measured as previously reported.22 Details of the assay are included in the supplemental Material and methods. Percent suppression was calculated by the formula: [(total frequency of proliferating CD4+ T cells cultured alone − frequency of proliferating CD4+ T cells after coculture with CB-derived B cells)/total frequency of proliferating CD4+ T cells cultured alone] × 100.
Transwell cultures
CB-derived fluorescence-activated cell sorter–sorted B-cell subsets and carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled allogeneic PB CFSE+CD4+ T cells (1 × 105) were cultured at a ratio of 1:1 either directly or separated in Transwell chambers (Millicell, 1.0 μm; Millipore) in the presence of anti-CD3/CD28 Dynabeads (Life Technologies). After 96 hours, cultured cells were harvested and analyzed by flow cytometry.
Blocking experiments
Purified B and T cells were cocultured and activated with anti-CD3/anti-CD28 Dynabeads in the presence or absence of blocking antibodies: anti-IL-10 (5 μg/mL; JEs#-9D7), anti-IL-10 receptor (IL-10R) (5 μg/mL; 3F9), anti-CD80 (10 μg/mL), anti-CD86 (10 μg/mL; IT2.2), anti-CTLA-4 (10 μg/mL; BNI3.1), or anti–transforming growth factor β (TGF-β) (2 μg/mL; TB21).
STAT3 signaling in B cells
STAT3 signaling was assessed as previously reported.22 Details of the assay are included in the supplemental Material and methods.
Statistics
Unless otherwise noted, all values are reported as medians and ranges. Statistical significance was performed with Prism (GraphPad) by unpaired or paired 2-tailed t test analysis and by nonparametric 2-way analysis of variance (ANOVA), as appropriate. A probability of P ≤ .05 was considered statistically significant.
Results
Human CB is enriched in IL-10–producing CD19+ B cells
Phenotypic characterization of CB revealed the presence of 2 distinct B-cell populations: CD19+CD38hiCD24hi transitional B cells (a population that includes immature B cells) and CD19+CD38intCD24int naive B cells (primarily mature B cells) (Figure 1A). Further extensive phenotypic characterization confirmed that the majority of CD19+CD38hiCD24hi transitional B cells were also IgMhiIgD+CD10+CD27−CD5hiCD1d+CD138−, whereas CD19+CD38intCD24int naive B cells were IgMintIgD+CD10−CD27−CD5intCD1d+CD138− (supplemental Figure 1), in keeping with previous reports.25,26,28,29 In contrast to PB, memory B cells and plasma cells were absent in CB (supplemental Figure 2).
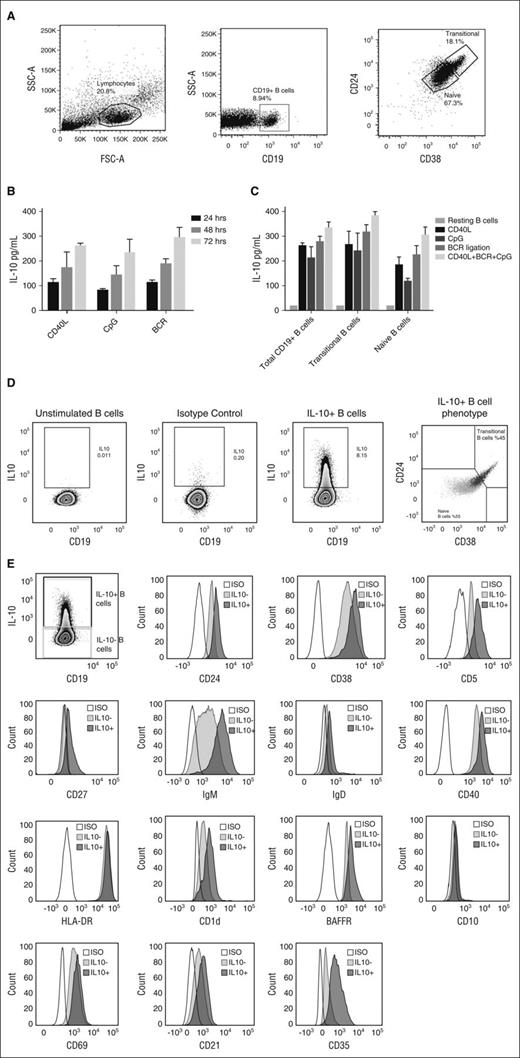
IL-10 production by CB-derived B cells after stimulation with CD40L, CpG, or BCR ligation. (A) Phenotypic characterization of cord blood B-cell subsets, as shown in representative fluorescence-activated cell sorter plots illustrating gating strategy on lymphocyte population, total CD19+ B cells and CD19+CD38hiCD24hi transitional B cells and CD19+CD38intCD24int naive B cells. (B) Bar graphs showing cumulative time-dependent IL-10 production by CB-derived CD19+ B cells in response to stimulation with CD40L, CpG, or BCR ligation (n = 10). CBMCs were stimulated with irradiated CD40L-transfected fibroblasts (L cells), CpG, or BCR ligation for 24, 48, or 72 hours. PMA (50 ng/mL) and ionomycin (250 ng/mL; Sigma-Aldrich) were added for the last 6 to 8 hours of the culture. Supernatants were harvested and assayed for IL-10 secretion by ELISA. (C) Cumulative IL-10 production by total CD19+ B cells vs sort-purified naive and transitional B-cell subsets after stimulation with CD40L, CpG, BCR ligation or their combination. Resting B cells (unstimulated) were used in each experiment as a negative control (n = 10). The bars in panels B and C represent the means and ranges. (D-E) CBMCs were stimulated with irradiated CD40L-transfected fibroblasts (L cells), BCR ligation, and CpG for 24 hours. PMA (50 ng/mL), ionomycin (250 ng/mL; Sigma-Aldrich), and brefeldin A (5 µg/mL; Sigma-Aldrich) were added for the last 6 to 8 hours of the culture. Cells were harvested, and surface stained with a cocktail of CD38 PEcy7 (eBioscience), HLA-DR FITC, CD40 PEcy7BAFFR FITC, CD22 FITC, CD23 PEcy7, CD21 FITC, CD25 PE, CD1d PE (all from Biolegend), CD24 FITC, CD27 PE, IgM PerCPcy5.5, IgD BV605, CD10 BV605, CD5 FITC, and CD43 FITC (all from BD Biosciences). Cells were fixed/permeabilized (eBioscience) and stained with APC-conjugated IL-10 or IgG2a-К isotype antibodies. Unstimulated B cells were also included as a negative control to validate IL-10 detection. All data were analyzed with FlowJo software. (D) Representative flow cytometry plots are shown for the distribution of IL-10+CD19+ B cells (black) and IL-10−CD19+ B cells (gray) on the CD24 v CD38 axis. lL-10+CD19+ B cells were enriched in both transitional (45%) and naive (55%) subsets. (E) Extended phenotyping of IL-10+ B cells (dark gray) vs IL-10− B cells (light gray) vs isotype control (white). APC, allophycocyanin; FITC, fluorescein isothiocyanate; FSC-A, forward scatter area; ISO, isotype control; PE, phycoerythrin; PerCP, peridinin chlorophyll; PMA, phorbol 12-myristate 13-acetate; SSC-A, side scatter area.
IL-10 production by CB-derived B cells after stimulation with CD40L, CpG, or BCR ligation. (A) Phenotypic characterization of cord blood B-cell subsets, as shown in representative fluorescence-activated cell sorter plots illustrating gating strategy on lymphocyte population, total CD19+ B cells and CD19+CD38hiCD24hi transitional B cells and CD19+CD38intCD24int naive B cells. (B) Bar graphs showing cumulative time-dependent IL-10 production by CB-derived CD19+ B cells in response to stimulation with CD40L, CpG, or BCR ligation (n = 10). CBMCs were stimulated with irradiated CD40L-transfected fibroblasts (L cells), CpG, or BCR ligation for 24, 48, or 72 hours. PMA (50 ng/mL) and ionomycin (250 ng/mL; Sigma-Aldrich) were added for the last 6 to 8 hours of the culture. Supernatants were harvested and assayed for IL-10 secretion by ELISA. (C) Cumulative IL-10 production by total CD19+ B cells vs sort-purified naive and transitional B-cell subsets after stimulation with CD40L, CpG, BCR ligation or their combination. Resting B cells (unstimulated) were used in each experiment as a negative control (n = 10). The bars in panels B and C represent the means and ranges. (D-E) CBMCs were stimulated with irradiated CD40L-transfected fibroblasts (L cells), BCR ligation, and CpG for 24 hours. PMA (50 ng/mL), ionomycin (250 ng/mL; Sigma-Aldrich), and brefeldin A (5 µg/mL; Sigma-Aldrich) were added for the last 6 to 8 hours of the culture. Cells were harvested, and surface stained with a cocktail of CD38 PEcy7 (eBioscience), HLA-DR FITC, CD40 PEcy7BAFFR FITC, CD22 FITC, CD23 PEcy7, CD21 FITC, CD25 PE, CD1d PE (all from Biolegend), CD24 FITC, CD27 PE, IgM PerCPcy5.5, IgD BV605, CD10 BV605, CD5 FITC, and CD43 FITC (all from BD Biosciences). Cells were fixed/permeabilized (eBioscience) and stained with APC-conjugated IL-10 or IgG2a-К isotype antibodies. Unstimulated B cells were also included as a negative control to validate IL-10 detection. All data were analyzed with FlowJo software. (D) Representative flow cytometry plots are shown for the distribution of IL-10+CD19+ B cells (black) and IL-10−CD19+ B cells (gray) on the CD24 v CD38 axis. lL-10+CD19+ B cells were enriched in both transitional (45%) and naive (55%) subsets. (E) Extended phenotyping of IL-10+ B cells (dark gray) vs IL-10− B cells (light gray) vs isotype control (white). APC, allophycocyanin; FITC, fluorescein isothiocyanate; FSC-A, forward scatter area; ISO, isotype control; PE, phycoerythrin; PerCP, peridinin chlorophyll; PMA, phorbol 12-myristate 13-acetate; SSC-A, side scatter area.
IL-10 production has long been considered a defining trait of Bregs.16 Thus, we determined whether CB-derived CD19+ B cells produce IL-10 by magnetically purifying CD19+ B cells from CB mononuclear cells (CBMCs) and culturing them with irradiated fibroblasts transfected with CD154 (L cells), cytosine guanine dinucleotide (CpG), or anti–B-cell receptor (BCR) for 24, 48, 72 hours and 5 days, after which we measured IL-10 production in the supernatants by enzyme-linked immunosorbent assay (ELISA) (Figure 1B). Our results confirmed that CB-derived CD19+ B cells have the capacity to produce IL-10 in response to stimulation in a time-dependent manner (Figure 1B; supplemental Figure 3).
To identify the source of the IL-10–producing B cells, we sort-purified naive and transitional B cells from healthy donor CB units and stimulated them with either L cells, CpG, BCR ligation, or a combination of the 3 for 48 hours. Interestingly, both naive and transitional CB-derived B cells produced comparable levels of IL-10 (Figure 1C). Moreover, stimulation of total CD19+ B cells and transitional and naive B-cell subsets with a combination of CD40 ligation, CpG, and BCR engagement led to significantly more IL-10 production than did culture with each stimulus alone (Figure 1C). To further define the phenotype of IL-10–producing CB-derived B cells, we combined intracellular staining for IL-10 with a panel of surface antibodies. IL-10–producing CB-B cells were enriched within both the CD19+CD38hiCD24hi transitional and CD19+CD38intCD24int naive B-cell subsets (Figure 1D). Furthermore, IL-10+CD19+ B cells expressed higher levels of CD24, CD38, CD5, CD27, IgM, CD1d, CD40, B-cell–activating factor belonging to the TNF family (BAFF) receptor (BAFFR), CD10, CD69, and CD25 when compared with IL-10− CB-B cells (Figure 1E). These results underscore the regulatory capacity of immature transitional B cells (CD19+CD24hiCD38hi), as previously shown by our group and others,18,21,22,30 and suggest that CB-derived naive B cells comprise a novel and previously undescribed subset of Bregs.
Sort-purified naive and transitional B cells inhibit proliferation and proinflammatory cytokine production by allogeneic peripheral blood CD4+ T cells in a dose-dependent manner
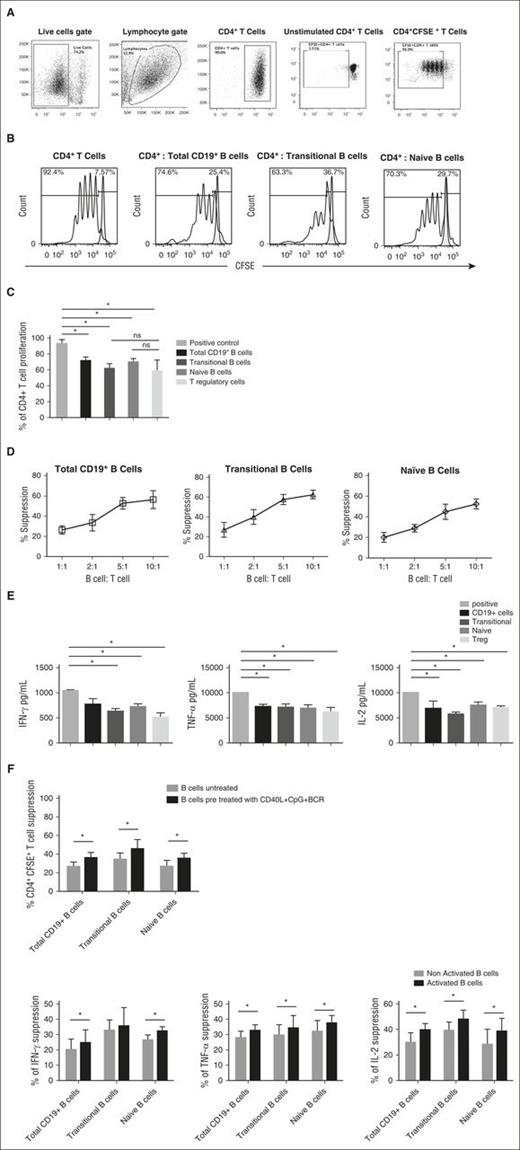
To gain further insight into the suppressive capacity of IL-10–enriched transitional and naive CB-derived B cells, we sort-purified these subsets as well as total CD19+ B cells from CB units (n = 10) and evaluated their suppressive effects on the proliferation and cytokine production of allogeneic PB CD4+ T cells. The gating strategies and postsort purity checks are outlined in supplemental Figure 4. After 96 hours of coculture with anti-CD3/anti-CD28-stimulated PB-CD4+ T cells at a ratio of 1:1, total CD19+ B cells and both naive and transitional B cells significantly suppressed the proliferation of allogeneic CD4+ T cells (Figure 2A-C), and these effects were cell dose-dependent (Figure 2D). Similarly, each B-cell subset significantly suppressed interferon-γ (IFN-γ), tumor necrosis factor (TNF-α), and IL-2 production by ex vivo–stimulated PB-derived CD4+ T cells following coculture (Figure 2E). On a per cell basis, the B cells from CB suppressed CD4+ T-cell proliferation and effector function to a lesser extent than did PB-derived transitional or IgM memory B cells (supplemental Figure 5).
IL-10–producing CB-derived CD19+ B cells and the naive and transitional B-cell subsets suppress CD4+ T-cell proliferation and effector function in a robust and dose-dependent manner. (A) Magnetically selected CD4+ T cells were labeled with CFSE (eBioscience) and plated in 96-well flat-bottomed tissue-culture plates. Total CD19+ B cells or sort-purified naive and transitional B-cell subsets were added to separate wells at a B-cell to T-cell ratio of 1:1 for 96 hours. T cells were activated with anti-CD3/CD28 Dynabeads (Invitrogen) as per the manufacturer’s instructions. CFSE-stained T cells cultured with no stimulation (negative control) and CFSE-stained T cells cultured with anti-CD3/anti-CD28 beads (positive proliferation control) were included in each experiment. Representative dot plots show the gating strategy for CD4+CFSE+ T cells. Gates were made on the live cells, lymphocyte population, followed by CD4+ T cells and CD4+CFSE+ T cells. Gating was determined with unstimulated CD4+ T cells. (B-C) Proliferation of CD4+ T cells cultured alone or with total CD19+ B cells, naive B cells, or transitional B cells at a ratio of 1:1. In vitro–suppressive effects of different CD19+ B-cell subsets cocultured with anti-CD3/anti-CD28–stimulated CD4+ T cells. Bars represent suppressive effects of CB-derived CD19+ B-cell subsets or CB Tregs (1:1 ratio) on CD4+ T-cell proliferation in vitro (n = 14). Bars represent median values and upper whiskers indicate the range. *P < .05 by nonparametric ANOVA. (D) Dose-dependent suppression of CD4+ T-cell proliferation in the presence of total CD19+ B cells or naive and transitional subsets cultured at the indicated B-cell to T-cell ratios. Data are plotted as the means and SEs of 4 independent experiments. (E). Bar graphs showing the suppression of IFN-γ, TNF-α, and IL-2 after coculture with CB-derived B-cell subsets and CB-Tregs. Bars indicate median values and upper whiskers represent range from 6 independent experiments. *P < .05 by nonparametric ANOVA. (F) Pretreatment of CB CD19+ B cells and B-cell subsets with CpG, CD40, or BCR ligation potentiated the suppressive effect of CB-derived B cells on CD4+ T-cell proliferation and effector function, by comparison with their noninduced counterparts (n = 4). Bars indicate median values and ranges (upper whiskers). *P < .05 by paired t test. ns, not significant; SE, standard error.
IL-10–producing CB-derived CD19+ B cells and the naive and transitional B-cell subsets suppress CD4+ T-cell proliferation and effector function in a robust and dose-dependent manner. (A) Magnetically selected CD4+ T cells were labeled with CFSE (eBioscience) and plated in 96-well flat-bottomed tissue-culture plates. Total CD19+ B cells or sort-purified naive and transitional B-cell subsets were added to separate wells at a B-cell to T-cell ratio of 1:1 for 96 hours. T cells were activated with anti-CD3/CD28 Dynabeads (Invitrogen) as per the manufacturer’s instructions. CFSE-stained T cells cultured with no stimulation (negative control) and CFSE-stained T cells cultured with anti-CD3/anti-CD28 beads (positive proliferation control) were included in each experiment. Representative dot plots show the gating strategy for CD4+CFSE+ T cells. Gates were made on the live cells, lymphocyte population, followed by CD4+ T cells and CD4+CFSE+ T cells. Gating was determined with unstimulated CD4+ T cells. (B-C) Proliferation of CD4+ T cells cultured alone or with total CD19+ B cells, naive B cells, or transitional B cells at a ratio of 1:1. In vitro–suppressive effects of different CD19+ B-cell subsets cocultured with anti-CD3/anti-CD28–stimulated CD4+ T cells. Bars represent suppressive effects of CB-derived CD19+ B-cell subsets or CB Tregs (1:1 ratio) on CD4+ T-cell proliferation in vitro (n = 14). Bars represent median values and upper whiskers indicate the range. *P < .05 by nonparametric ANOVA. (D) Dose-dependent suppression of CD4+ T-cell proliferation in the presence of total CD19+ B cells or naive and transitional subsets cultured at the indicated B-cell to T-cell ratios. Data are plotted as the means and SEs of 4 independent experiments. (E). Bar graphs showing the suppression of IFN-γ, TNF-α, and IL-2 after coculture with CB-derived B-cell subsets and CB-Tregs. Bars indicate median values and upper whiskers represent range from 6 independent experiments. *P < .05 by nonparametric ANOVA. (F) Pretreatment of CB CD19+ B cells and B-cell subsets with CpG, CD40, or BCR ligation potentiated the suppressive effect of CB-derived B cells on CD4+ T-cell proliferation and effector function, by comparison with their noninduced counterparts (n = 4). Bars indicate median values and ranges (upper whiskers). *P < .05 by paired t test. ns, not significant; SE, standard error.
We further compared the suppressive capacity of 3 CB-derived B-cell populations, (ie, total CD19+ B cells and naive and transitional B cells) with that of CB-derived Tregs, defined as CD4+CD25hiCD127− T cells. In experiments shown in Figure 2, panels C and E, T-cell proliferation and IFN-γ, TNF-α, and IL-2 production were inhibited to a similar extent by the B-cell subsets and Tregs. Collectively, these results demonstrate that on a per-cell basis, CB-derived Bregs share with Tregs a similar potential to suppress CD4+ T-cell expansion and cytokine production. Finally, pretreatment of the B cells with CpG, CD40, and BCR ligation to induce IL-10 production potentiated their suppressive capacity beyond results achieved with their “nonactivated” counterparts (Figure 2F).
IL-10 contributes to the immunoregulatory function of CB-derived transitional and naive B cells
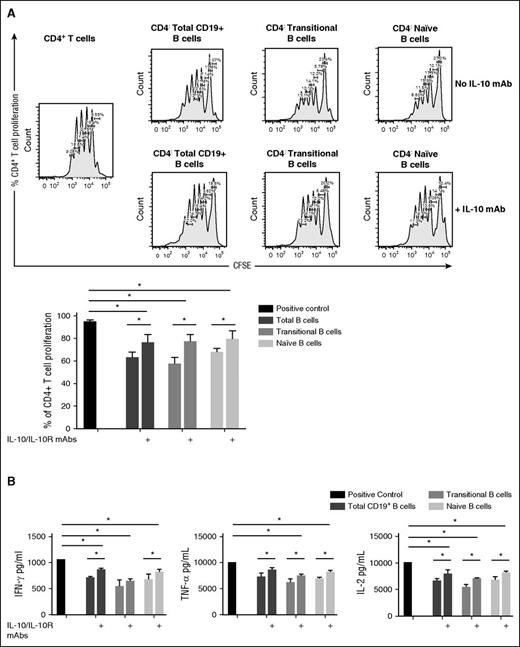
How might CB-derived B cells suppress CD4+ T-cell proliferation and effector cytokine function? To address this issue, we cultured allogeneic CD4+ T cells from PB either alone or with total CD19+ B cells or naive or transitional B-cell subsets in the presence or absence of blocking monoclonal antibodies (mAbs) against IL-10 and IL-10R, based on reports linking IL-10 secretion to the suppressive capabilities of Bregs in mice and human PB.18,21,22,30 Addition of blocking antibodies against IL-10 or IL-10R individually or in combination could equally reverse the suppressive activity of CB B-cell subsets (supplemental Figure 6). IL-10/IL-10R blockade partially restored the proliferation and cytokine production of CD4+ T cells cocultured with total CB-derived CD19+ B cells, or the naive or transitional B-cell subsets at a 1:1 ratio (Figure 3). These results implicate IL-10 as a mediator of the regulatory properties of CB-derived Bregs, but do not exclude other mechanisms such as TGF-β, which has been described to mediate Breg suppression in a number of autoimmune murine models.31 However, in additional blocking experiments with TGF-β–specific mAbs, there was no evidence to support a role for this cytokine in CB-derived Breg-mediated inhibition of peripheral T cells (supplemental Figure 7).
Suppressive activity of CB-derived total CD19+ B cells and naive and transitional B-cell subsets partly depends on IL-10 secretion. (A) Suppressive effect of B-cell subsets on proliferation of CFSE-labeled CD4+ T cells in the presence or absence of IL-10 blockade. Flow cytometry histograms show CD4+ T cells cultured alone or with total CD19+ B cells, naive B cells, or transitional B cells at a 1:1 ratio in the presence or absence of a blocking mAb to both IL-10 and IL-10R simultaneously. Data represent 4 independent experiments. Bars indicate median values and upper whisker of error bar represent range. *P < .05 by nonparametric ANOVA. (B) IL-10 blockade partially reverses the suppressive effect of CB-derived B cells on CD4+ T-cell cytokine secretion at a 1:1 (B cell:T-cell) ratio. Supernatants were harvested from B-cell/T-cell cocultures and assayed for the presence of IL-2, IFN-γ, and TNF-α production by ELISA. Data are representative of 4 independent experiments. Bars indicate median values and ranges (upper whiskers). *P < .05 by nonparametric ANOVA.
Suppressive activity of CB-derived total CD19+ B cells and naive and transitional B-cell subsets partly depends on IL-10 secretion. (A) Suppressive effect of B-cell subsets on proliferation of CFSE-labeled CD4+ T cells in the presence or absence of IL-10 blockade. Flow cytometry histograms show CD4+ T cells cultured alone or with total CD19+ B cells, naive B cells, or transitional B cells at a 1:1 ratio in the presence or absence of a blocking mAb to both IL-10 and IL-10R simultaneously. Data represent 4 independent experiments. Bars indicate median values and upper whisker of error bar represent range. *P < .05 by nonparametric ANOVA. (B) IL-10 blockade partially reverses the suppressive effect of CB-derived B cells on CD4+ T-cell cytokine secretion at a 1:1 (B cell:T-cell) ratio. Supernatants were harvested from B-cell/T-cell cocultures and assayed for the presence of IL-2, IFN-γ, and TNF-α production by ELISA. Data are representative of 4 independent experiments. Bars indicate median values and ranges (upper whiskers). *P < .05 by nonparametric ANOVA.
To uncover potential mechanisms for the observed differences in the suppressive capacity of PB- vs CB-naive B cells, we measured the level of phosphorylated STAT3 (pSTAT3) expression upon CD40 stimulation in PB and CB B-cell subsets. Although the level of pSTAT3 expression was comparable between transitional B cells from both sources, CD40 ligation induced higher pSTAT3 levels in the CB-naive compared with PB-naive B cells, suggesting that the former may have a lower threshold of activation and therefore may be more competent to secrete IL-10 (supplemental Figure 8). Indeed, these results correlated with the levels of IL-10 measured in the supernatants of activated B cells, where CB-naive B cells secreted higher levels of IL-10 than PB-naive B cells (supplemental Figure 9). Thus, because IL-10 has been implicated as a mediator of CB-Breg–suppressive function,13 this mechanistic insight may help to explain why PB- and CB-derived naive B cells differ in their suppressive capacity.
Suppressive activity of CB-derived Bregs partly depends on cell-to-cell contact, mediated through CD80/86 and CTLA-4
The suppressive capability of both murine and human PB-derived Bregs has been linked to direct contact with CD4+ T cells,18,21,22 but whether this mechanism contributes to T-cell suppression by CB-derived Bregs remains uncertain. Thus, we performed Transwell experiments in which total CD19+, transitional, and naive B cells were either in direct contact or separated from anti-CD3/anti-CD28-stimulated and CFSE-stained PB-derived CD4+ T cells by a permeable membrane. Direct coculture of CFSE-stained CD4+ T cells with each of the 3 B-cell subsets resulted in significant suppression of CD4+ T-cell proliferation (Figure 4A). By contrast, separation of CB-derived B cells and CD4+ T cells by a Transwell membrane partially reversed the suppressive effect of the CB-derived Bregs at a 1:1 ratio (Figure 4A). Similarly, separation of the Bregs from CD4+ T cells by a Transwell membrane partially reversed their ability to suppress IFN-γ, TNF-α, and IL-2 production by ex vivo–activated CD4+ T cells (Figure 4B). To examine whether soluble CD40L, which is naturally secreted by activated T cells, can function as the trigger for IL-10 production by B cells in the Transwell setting, we measured the level of this soluble factor by ELISA in supernatants harvested from Transwell cultures. As shown in supplemental Figure 10, soluble CD40L was present in the cocultures with CD3/CD28-activated CD4+ T cells. Thus, we propose that soluble CD40L secreted by activated T cells can cross the Transwell membrane and induce IL-10 production by CB-derived B cells to mediate T-cell suppression.
Direct cell-cell contact contributes to the T-cell–suppressive activity of CB-derived B cells. (A) Representative histograms showing proliferation of CFSE-stained anti-CD3/anti-CD28-stimulated CD4+ T cells cultured alone (positive control) or in direct contact with CB-B cells (direct contact) or separated from CB-B cells by Transwell chambers (Transwell). For each of these conditions, CD4+ T cells were cultured at a 1:1 ratio with CB-derived total B cells or sort-purified naive or transitional B-cell subsets. Bar graphs illustrate collective data representative from 4 independent experiments. (B) Effect of B:T cell-to-cell contact on CD4+ T-cell cytokine production. Anti-CD3/anti-CD28-stimulated CD4+ T cells were cultured alone (positive control) or in direct contact with CB-B cells (direct contact) or separated from CB-B cells by Transwell chambers (Transwell). For each of these conditions, CD4+ T cells were cultured at a 1:1 ratio with CB-derived total B cells or sort-purified naive or transitional B-cell subsets. Bar graphs illustrate pooled data from 4 independent experiments, comparing the suppressive activity of CB-derived B cells in the presence or absence of direct cell-cell on T-cell cytokine production measured by intracellular cytokine staining and ELISA. (C-D) Effect of B:T-cell-to-cell and IL-10 blocking on CD4+ T-cell proliferation (n = 4) (C) and cytokine production (n = 4) (D). Anti-CD3/anti-CD28-stimulated CD4+ T cells were cultured alone (positive control) or in direct contact with CB-derived B cells or separated from them by a Transwell chamber in the presence or absence of blocking antibodies against both IL-10 and IL-10R. Bar graphs illustrate collective data from 4 independent experiments. Bars indicate median values and ranges (upper whiskers). *P < .05 by nonparametric ANOVA.
Direct cell-cell contact contributes to the T-cell–suppressive activity of CB-derived B cells. (A) Representative histograms showing proliferation of CFSE-stained anti-CD3/anti-CD28-stimulated CD4+ T cells cultured alone (positive control) or in direct contact with CB-B cells (direct contact) or separated from CB-B cells by Transwell chambers (Transwell). For each of these conditions, CD4+ T cells were cultured at a 1:1 ratio with CB-derived total B cells or sort-purified naive or transitional B-cell subsets. Bar graphs illustrate collective data representative from 4 independent experiments. (B) Effect of B:T cell-to-cell contact on CD4+ T-cell cytokine production. Anti-CD3/anti-CD28-stimulated CD4+ T cells were cultured alone (positive control) or in direct contact with CB-B cells (direct contact) or separated from CB-B cells by Transwell chambers (Transwell). For each of these conditions, CD4+ T cells were cultured at a 1:1 ratio with CB-derived total B cells or sort-purified naive or transitional B-cell subsets. Bar graphs illustrate pooled data from 4 independent experiments, comparing the suppressive activity of CB-derived B cells in the presence or absence of direct cell-cell on T-cell cytokine production measured by intracellular cytokine staining and ELISA. (C-D) Effect of B:T-cell-to-cell and IL-10 blocking on CD4+ T-cell proliferation (n = 4) (C) and cytokine production (n = 4) (D). Anti-CD3/anti-CD28-stimulated CD4+ T cells were cultured alone (positive control) or in direct contact with CB-derived B cells or separated from them by a Transwell chamber in the presence or absence of blocking antibodies against both IL-10 and IL-10R. Bar graphs illustrate collective data from 4 independent experiments. Bars indicate median values and ranges (upper whiskers). *P < .05 by nonparametric ANOVA.
The above results suggest that it might be possible to reverse the suppressive effects of CB-derived Bregs on CD4+ T-cell proliferation by combining an IL-10 blockade with the abrogation of cell-cell contact. To test this prediction, we added IL-10/IL-10R–blocking mAbs to either purified total CB-CD19+ B cells or transitional and naive B cells in a Transwell setting. As shown in Figure 4C-D, this experiment completely abolished the suppressive effect of CB-derived B-cell subsets on CD4+ T-cell proliferation and cytokine production.
Prompted by evidence from both murine and human B-cell experimental systems,21,29 we next tested the contribution of CD80 and CD86 costimulatory signaling to the suppressive capacity of sort-purified CB-derived total CD19+ B cells, as well as the transitional and naive B-cell subsets. Although the addition of blocking antibodies against CD80 or CD86 molecules individually was not sufficient to reverse the suppressive activity of CB-Bregs (data not shown), addition of blocking antibodies against both molecules partially inhibited the ability of CB-derived Bregs to suppress the effector function and proliferation of PB-CD4+ T cells (Figure 5A). This suggests that the suppressive effect of the Breg cells is at least partially mediated by CD80/CD86 costimulatory signaling. Because CD80/CD86 molecules interact with the CTLA-4 inhibitory receptor on T cells,32,33 we also considered that the suppressive activity of CB-derived Bregs may depend on CD80/CD86 interaction with CTLA-4. The addition of a blocking antibody against CTLA-4 partially inhibited the ability of CB-derived B-cell subsets to suppress peripheral CD4+ T function (Figure 5B-C). This effect was enhanced when we combined CTLA-4 with CD80/CD86 blockade (Figure 5D). These results suggest an important interaction between CD80/CD86 on CB-derived Bregs and CTLA-4 on CD4+ T cells in CB-Breg–mediated T-cell suppression. Although the blockade of IL-10/IL-10R, CD80/CD86, and CTLA-4 individually was not sufficient to abolish the suppressive capacity of regulatory CB-derived total CD19+ B cells or the transitional and naive B-cell subsets, it was possible to achieve this endpoint with a combination of mAbs against all target molecules (Figure 5D).
CD80/CD86 and CTLA-4 coreceptor signaling is a prerequisite for the suppressive effect of CB-derived Bregs. (A) CD80/86 blockade significantly inhibits the ability of CB B-cell subsets to suppress the effector function and proliferation of peripheral CD4+ T cells. Cumulative data show the effect of CD80 and CD86 coreceptor blockade in cultures of purified CFSE-stained proliferating CD4+ T cells and sorted CB-derived CD19+ B-cell subsets at a 1:1 ratio. Bar graphs illustrate collective data from 4 independent experiments. (B) CTLA-4 blockade significantly inhibits the ability of CB B-cell subsets to suppress the effector function and proliferation of peripheral CD4+ T cells. The effects of CTLA-4 blockade were assessed in cultures of purified CFSE-stained proliferating CD4+ T cells and sorted CB-derived CD19+ B-cell subsets as compared with the corresponding positive control. Bar charts compare the effect of CTLA-4 blocking on CD4+ T-cell proliferation and IFN-γ, TNF-α, and IL-2 production at a 1:1 B-cell to T-cell ratio (n = 4). (C) CTLA-4 blockade has no significant impact on the ability of PB-derived Breg subsets to suppress the proliferation of peripheral CD4+ T cells. The effects of CTLA-4 blockade were assessed in cultures of purified CFSE-stained proliferating CD4+ T cells and sort-purified PB-derived CD19+ B-cell subsets as compared with the corresponding positive control (n = 4). (D) A combination of blocking antibodies to IL-10, CTLA-4, CD80, and CD86 is sufficient to fully reverse the ability of CB-Breg subsets to suppress CD4+ T-cell proliferation in vitro (n = 4). Bars indicate median values and ranges (upper whiskers). *P < .05 by nonparametric ANOVA.
CD80/CD86 and CTLA-4 coreceptor signaling is a prerequisite for the suppressive effect of CB-derived Bregs. (A) CD80/86 blockade significantly inhibits the ability of CB B-cell subsets to suppress the effector function and proliferation of peripheral CD4+ T cells. Cumulative data show the effect of CD80 and CD86 coreceptor blockade in cultures of purified CFSE-stained proliferating CD4+ T cells and sorted CB-derived CD19+ B-cell subsets at a 1:1 ratio. Bar graphs illustrate collective data from 4 independent experiments. (B) CTLA-4 blockade significantly inhibits the ability of CB B-cell subsets to suppress the effector function and proliferation of peripheral CD4+ T cells. The effects of CTLA-4 blockade were assessed in cultures of purified CFSE-stained proliferating CD4+ T cells and sorted CB-derived CD19+ B-cell subsets as compared with the corresponding positive control. Bar charts compare the effect of CTLA-4 blocking on CD4+ T-cell proliferation and IFN-γ, TNF-α, and IL-2 production at a 1:1 B-cell to T-cell ratio (n = 4). (C) CTLA-4 blockade has no significant impact on the ability of PB-derived Breg subsets to suppress the proliferation of peripheral CD4+ T cells. The effects of CTLA-4 blockade were assessed in cultures of purified CFSE-stained proliferating CD4+ T cells and sort-purified PB-derived CD19+ B-cell subsets as compared with the corresponding positive control (n = 4). (D) A combination of blocking antibodies to IL-10, CTLA-4, CD80, and CD86 is sufficient to fully reverse the ability of CB-Breg subsets to suppress CD4+ T-cell proliferation in vitro (n = 4). Bars indicate median values and ranges (upper whiskers). *P < .05 by nonparametric ANOVA.
Suppressive activity of naive and transitional CB-derived B cells does not depend on Treg activity in vitro
To test whether the suppressive effects of IL-10–producing B-cell subsets from CB are partly mediated by Tregs, we depleted CD25+CD127− Tregs from CD4+ T cells by using magnetic bead cell purification. The resultant Treg-depleted CD4+ T cells were then CFSE-stained, stimulated with anti-CD3/anti-CD28-stimulated, and cultured at a 1:1 ratio with CB-derived B-cell subsets. Each of the 3 CD19+ B-cell populations (naive, transitional, and total) significantly suppressed the proliferation of Treg-depleted CD4+ T cells (supplemental Figure 11).
Bregs in CB may account for lower rates of cGVHD after CBT
Rapid B-cell recovery following allo-HSCT has been reported to correlate with lower rates of cGVHD.4.9,12 Thus, given the ability of CB-derived CD19+ B-cell subsets to control CD4+ T-cell function, we hypothesized that higher frequencies of Bregs in CB grafts contribute to the lower rate of cGVHD development post-CBT. To test this hypothesis, we next studied the recovery of IL-10+ B cells in 27 CB recipients at various time points of pretransplant, at 1 month, at intervals of 90 days for up to 1 year and then at 2 years post-CBT (Table 1). Whereas B cells collected from patients at 1 to 3 months post-CBT expressed low IL-10 levels when activated by CD40L, significantly elevated absolute counts and frequencies of CD19+IL-10+ B cells were apparent in CB recipients at 3 to 9 months (Figure 6A-B; supplemental Figure 12). Indeed, the frequency of CD19+IL-10+ B cells was significantly higher in CB recipients at this time interval than in either PB or CB samples from healthy donors (Figure 6C), but declined progressively after 9 months post-CBT, to levels comparable to those in healthy individuals. Based on the chimerism data (supplemental Table 1), the recovering Bregs after CBT were mostly donor-derived. The early and robust reconstitution of the IL-10+ B-cell pool post-CBT supports an important role for donor CB-derived B cells in protection against cGVHD.
B cells from patients undergoing CBT show an early and robust reconstitution of both the CD19+ B cells and the IL-10+ B-cell pools. (A) IL-10 secretion by total CD19+ B cells. PBMCs collected from 27 CBT patients and cultured with CD40L for 48 hours were stained for the CD19+IL-10+ phenotype by intracellular flow cytometric staining. The samples represented the following collection time points: pretransplant (n = 19), 1 month (n = 20), 3 months (n = 22), 6 months (n = 16), 9 months (n = 12), 1 year (n = 12) and 2 years (n = 8) post-CBT. Supernatants were also harvested from cultures of activated patient-derived B cells and assayed for IL-10 secretion by ELISA from samples collected at the following times: pretransplant (n = 12), 1 month (n = 10), 3 months (n = 10), 6 months (n = 10), 9 months (n = 8), 1 year (n = 12), and 2 years (n = 8) post-CBT. Bars indicate mean values and ranges (whiskers). (B) Absolute counts of IL-10+CD19+ B cells in PBMCs collected from 27 CB recipients, available at the following time points: pretransplant (n = 19), 1 month (n = 20), 3 months (n = 22), 6 months (n = 16), 9 months (n = 12), 1 year (n = 12) and CD19+B cells at 2 years (n = 8) post-CBT. (C) Comparison of IL-10 secretion by activated total CD19+ B cells in PB (n = 10) and CB (n = 10) from healthy donors and patients at 6 months posttransplant (n = 10). Bars denote median values and ranges (whiskers). *P < .05 by nonparametric ANOVA. (D) CD19+ B-cell frequencies and absolute counts per microliter in patients with cGVHD (n = 12) compared with patients without cGVHD (n = 15). *P < .05 and **P < .01 by unpaired t test. (E) CD19+IL-10+ B-cell frequencies and absolute counts per microliter in patients with cGVHD (n = 12) compared with patients without cGVHD (n = 15). *P < .05 and **P < .01 by unpaired t test. In both panels D and E, samples from patients with GVHD were available at the following time points: pretransplant (n = 9), 1 month (n = 10), 3 months (n = 10), 6 months (n = 7), 9 months (n = 6), 1 year (n = 5), and 2 years (n = 3) post-CBT. Samples from patients without GVHD were available at the following time points: pretransplant (n = 10), 1 month (n = 10), 3 months (n = 12), 6 months (n = 9), 9 months (n = 6), 1 year (n = 7), and 2 years (n = 5) post-CBT. The data points are mean values with ranges (whiskers). (F) Reconstituting IL-10–producing regulatory CD19+ B cells from patients possess greater suppressive function on T cells as compared with healthy CB B cells or PB B cells from healthy donors on a cell-per-cell basis. CD19+ B cells magnetically isolated from post-CBT patients at different time points post-CBT were cultured at a 1:1 ratio with anti-CD3/anti-CD28-activated CFSE+CD4+ T cells from healthy individuals for 96 hours, and then harvested and stained for CD4+CFSE+ proliferating T cells (healthy cord blood CD19+ B cells [n = 12]; CD19+ B cells at 1 month [n = 3]; CD19+ B cells at 3 months [n = 5]; CD19+ B cells at 6 months [n = 8]; CD19+ B cells at 9 months [n = 7]; CD19+ B cells at 1 year [n = 8]; CD19+ B cells at 2 years [n = 3]). (G) Patients with cGVHD (n = 6) had higher frequencies and absolute numbers of IFN-γ+CD4+ T cells compared with those without cGVHD (n = 4). (H) Patients with cGVHD (n = 6) had a reduced ratio of IL-10+ B cells to IFN-γ+CD4+ T cells compared with patients without cGVHD (n = 4). In both panels, bars denote mean values and ranges (whiskers). *P < .05 by unpaired t test. PBMC, peripheral blood mononuclear cell.
B cells from patients undergoing CBT show an early and robust reconstitution of both the CD19+ B cells and the IL-10+ B-cell pools. (A) IL-10 secretion by total CD19+ B cells. PBMCs collected from 27 CBT patients and cultured with CD40L for 48 hours were stained for the CD19+IL-10+ phenotype by intracellular flow cytometric staining. The samples represented the following collection time points: pretransplant (n = 19), 1 month (n = 20), 3 months (n = 22), 6 months (n = 16), 9 months (n = 12), 1 year (n = 12) and 2 years (n = 8) post-CBT. Supernatants were also harvested from cultures of activated patient-derived B cells and assayed for IL-10 secretion by ELISA from samples collected at the following times: pretransplant (n = 12), 1 month (n = 10), 3 months (n = 10), 6 months (n = 10), 9 months (n = 8), 1 year (n = 12), and 2 years (n = 8) post-CBT. Bars indicate mean values and ranges (whiskers). (B) Absolute counts of IL-10+CD19+ B cells in PBMCs collected from 27 CB recipients, available at the following time points: pretransplant (n = 19), 1 month (n = 20), 3 months (n = 22), 6 months (n = 16), 9 months (n = 12), 1 year (n = 12) and CD19+B cells at 2 years (n = 8) post-CBT. (C) Comparison of IL-10 secretion by activated total CD19+ B cells in PB (n = 10) and CB (n = 10) from healthy donors and patients at 6 months posttransplant (n = 10). Bars denote median values and ranges (whiskers). *P < .05 by nonparametric ANOVA. (D) CD19+ B-cell frequencies and absolute counts per microliter in patients with cGVHD (n = 12) compared with patients without cGVHD (n = 15). *P < .05 and **P < .01 by unpaired t test. (E) CD19+IL-10+ B-cell frequencies and absolute counts per microliter in patients with cGVHD (n = 12) compared with patients without cGVHD (n = 15). *P < .05 and **P < .01 by unpaired t test. In both panels D and E, samples from patients with GVHD were available at the following time points: pretransplant (n = 9), 1 month (n = 10), 3 months (n = 10), 6 months (n = 7), 9 months (n = 6), 1 year (n = 5), and 2 years (n = 3) post-CBT. Samples from patients without GVHD were available at the following time points: pretransplant (n = 10), 1 month (n = 10), 3 months (n = 12), 6 months (n = 9), 9 months (n = 6), 1 year (n = 7), and 2 years (n = 5) post-CBT. The data points are mean values with ranges (whiskers). (F) Reconstituting IL-10–producing regulatory CD19+ B cells from patients possess greater suppressive function on T cells as compared with healthy CB B cells or PB B cells from healthy donors on a cell-per-cell basis. CD19+ B cells magnetically isolated from post-CBT patients at different time points post-CBT were cultured at a 1:1 ratio with anti-CD3/anti-CD28-activated CFSE+CD4+ T cells from healthy individuals for 96 hours, and then harvested and stained for CD4+CFSE+ proliferating T cells (healthy cord blood CD19+ B cells [n = 12]; CD19+ B cells at 1 month [n = 3]; CD19+ B cells at 3 months [n = 5]; CD19+ B cells at 6 months [n = 8]; CD19+ B cells at 9 months [n = 7]; CD19+ B cells at 1 year [n = 8]; CD19+ B cells at 2 years [n = 3]). (G) Patients with cGVHD (n = 6) had higher frequencies and absolute numbers of IFN-γ+CD4+ T cells compared with those without cGVHD (n = 4). (H) Patients with cGVHD (n = 6) had a reduced ratio of IL-10+ B cells to IFN-γ+CD4+ T cells compared with patients without cGVHD (n = 4). In both panels, bars denote mean values and ranges (whiskers). *P < .05 by unpaired t test. PBMC, peripheral blood mononuclear cell.
We next examined the differences in CD19+ B-cell and CD19+IL-10+ B-cell recovery post-CBT between patients who developed cGVHD (n = 12) vs those who lacked this complication (n = 15). Patients with cGVHD had significantly lower frequencies and absolute counts per microliter of CD19+ B cells and IL-10–producing CD19+ B cells during 3 to 9 months post-CBT, compared with results for patients without GVHD (Figure 6D-E), despite the similar overall frequencies of transitional and naive B cells in these 2 groups of patients (data not shown).
To confirm that the recovering B cells after CBT possess regulatory function and can suppress effector T-cell function, we magnetically isolated CD19+ B cells from the PB of CB recipients with available samples at different times posttransplant and cultured them at a 1:1 ratio with anti-CD3/anti-CD28-activated CD4+ T cells from HLA-mismatched allogeneic healthy donors. CD19+ B cells isolated from the PB of CB recipients at 3 months or later after transplant effectively suppressed proliferation by allogeneic CD4+CD25− T cells (Figure 6F). The suppressive capacity of B cells collected from patients 6 to 9 months post-CBT was superior to that of PB- or CB-derived B cells from healthy controls. Our results indicate the presence of an expanded population of IL-10–producing regulatory CD19+ B cells at 6 to 9 months post-CBT, supporting a role for CB-derived Bregs in limiting or preventing the severity of GVHD.
An imbalance between PB-derived Bregs and effector T cells has been reported in patients with GVHD.18 Thus, we explored whether the functional deficit noted in the recovery of IL-10+CD19+ B cells post-CBT may contribute to an altered ratio of regulatory-to-effector cells in CB-transplant recipients with cGVHD. The frequency and absolute numbers of IFN-γ+CD4+ T cells was significantly elevated in CBT recipients with (n = 6) compared with those without (n = 4) cGVHD (Figure 6G). Additionally, patients with cGVHD had a significantly higher IFN-γ+CD4+/IL-10+CD19+ B-cell ratio, both in terms of relative frequency and absolute counts than did patients without GVHD (Figure 6H). These results suggest an imbalance between the regulatory and proinflammatory networks in patients with cGVHD.
Discussion
Immune regulation is governed by the dynamic equilibrium between effector T-cell responses and tolerance-mediating regulatory pathways, a balance that has been largely ascribed to Tregs.9 The pathophysiology of cGVHD involves impaired regulatory mechanisms of tolerance between recipient tissues and donor-derived immunity, but our understanding of the function of Bregs in this disease remains limited. Recent research has identified a functional group of IL-10–producing CD19+CD24hiCD38hi transitional B cells in human PB that possess immune-regulatory capacity.22 Conversely, others have described human IL-10–producing B cells that reside predominantly within the CD24hiCD27+ memory B-cell compartment.19,23,24 Khoder et al have shown that regulatory subsets of IL-10–secreting CD19+IgM+CD27+ memory B cells coexist with IL-10+CD24hiCD38hi transitional B cells in healthy human donors and play a role in protection against cGVHD after HSCT.18 Finally, recent evidence establishes the presence of CD19+CD38hiCD24hi immature transitional B cells that are abundant in human CB.25-28 Here, we demonstrate the regulatory capacity of CB-derived CD19+CD38hiCD24hi transitional B cells and CD19+CD38intCD24int naive B cells on peripheral CD4+ T-cell proliferation and effector function. Unlike PB-naive B cells that failed to exert suppressive activity on CD4+ T cells,18 we found that CB-naive B cells are suppressive. Notably, CB naive cells are enriched in late transitional B cells, including T3 cells, and are less mature than PB naive B cells.29 Thus, it is possible that they may have retained some of the regulatory characteristics of transitional B cells. We further explored differences between the suppressive capacity of PB-naive and CB-naive B cells and found that the latter expressed higher levels of pSTAT3 upon CD40 engagement than did PB-naive B cells and were more prone to secrete IL-10, suggesting that they may have a lower threshold of activation that could mediate their suppressive function.
We further demonstrate that the suppressive capacity of CB-derived Bregs against CD4+ T cells is potentiated after preactivation, suggesting that in human PB, the Breg designation may not be limited to the memory and transitional B-cell compartment, as previously described.18,22-24 We think it likely that discrete subsets of naive and switched memory B cells could also be induced to exert regulatory function in response to CD40L signaling provided by activated T cells, consistent with reports of Treg induction during inflammation.34-36
As in previous studies of PB-derived Bregs,18,22 the suppressive mechanism of CB- derived Bregs was mediated synergistically through IL-10 production and cell-to-cell contact. IL-10 blockade partially reversed CD4+ T-cell proliferation and effector cytokine secretion in the presence of total CD19+ or naive or transitional B cells. In contrast to murine Breg studies,37 we did not find a significant role for TGF-β in T-cell suppression mediated by CB-derived Bregs. Additional mechanistic studies using Transwell chambers and CD80/86 blockade confirmed that CD80/CD86 interactions between B cells and CD4+ T cells cooperate with B-cell IL-10 production to ensure a full suppressive effect on CD4+ T-cell function. Our findings agree with human PB-derived studies in which the involvement of CD80/CD86 is an important feature of Breg-suppressive capacity,18,22 and with murine studies of intestinal inflammation where CD86, in particular, has been noted to facilitate B-cell suppression.13,38 Conversely, the involvement of CD80/CD86 interaction with CTLA-4 on T cells as part of the overall suppressive mechanism of CB-derived Bregs adds a new dimension to the regulatory function of these cells, as PB-derived Bregs do not appear to require interaction with CTLA-4 to mediate suppression.18
The role of Bregs in CBT and GVHD remains largely undefined. Several groups have reported that immune reconstitution after CBT is characterized by an expansion in B cells during the first year posttransplant.4,5,31,39,40 In our analysis of B-cell reconstitution in 27 CB recipients, B cells could be detected at low frequencies as early as 1 month post-CBT, when the majority of B cells had a CD24hiCD38hi transitional profile. There was further expansion in the frequencies and absolute numbers of CD19+ B cells between 3 and 9 months, after which the B-cell population decreased progressively until 1 year post-CBT, when these endpoints were similar to findings in healthy donors. Our data further illustrate a similar trend in the kinetics of IL-10–producing CD19+ B-cell reconstitution post-CBT. Importantly, CB recipients who developed GVHD had a reduced CD19+ B-cell recovery at all time points studied by comparison with recipients who did not develop this complication. In addition, CD19+ B cells isolated from CB recipients with GVHD were refractory to stimulation and had a significantly slower reconstitution of CD19+IL-10+ B cells at 3 to 9 months post-CBT, relative to that in patients without GVHD. These results are in line with a similar study documenting low frequencies of IL-10–producing B cells in cGVHD vs no cGVHD patients undergoing HSCT.18 Moreover, we directly demonstrate the ability of CD19+ B cells post-CBT to suppress T-cell function in vitro. Indeed, B cells collected from patients at 6 to 9 months post-CBT had an even greater suppressive capacity than those from PB or CB healthy donors. We also observed a significantly lower IL-10+ B-cell to IFN-γ+CD4+ T-cell ratio in cGVHD patients compared with the no-cGVHD group, which implies an imbalance between the Breg and effector T-cell compartments, analogous to that recognized with PB-Bregs18 and Tregs during the development of cGVHD.41 Our findings therefore support a broader than expected deficit in the immune-regulated network that is involved in the control of cGVHD. We hypothesize from these results that the early recovery of B cells post-CBT may indicate a protective role for Bregs in the GVHD setting. However, it is possible that other factors may also contribute to Breg recovery and the risk of cGVHD, such as the impact of myeloblative vs reduced-intensity conditioning regimens, reported to influence BAFF levels and B-cell recovery.42 Environmental stimuli within the recipient and differences in the recovery of other reconstituting cell populations may also impact Breg recovery post-CBT. A recent elegant study by Mauri’s group highlighted the important role of plasmacytoid dendritic cells in induction of Breg differentiation.43 Moreover, CD40 signaling cascades22,44 as well as proinflammatory cytokines such as IL-1β, IL-6,45,46 and IL-2147 can also induce the differentiation of IL-10–producing Bregs. Hence, the role of inflammation and identification of stimuli that can induce Bregs is important to consider in patients with cGVHD.
Unfortunately, given the lack of methods to purify IL-10–producing B cells in sufficient quantities for functional studies, we are currently unable to pursue this or other potential explanations for a protective effect of Bregs. Thus, it will be critical to search for a unique Breg signature that would reliably identify IL-10–producing B cells with immunoregulatory capacity.
The potential role of CB-derived donor Bregs delineated by this study may lead to new B-cell–directed therapies for GVHD that specifically target B-cell reconstitution and function posttransplant. In this approach, it will be necessary to understand whether lower Breg frequencies in patients with cGVHD are due to a deficiency in donor Bregs or secondary to defective immune and inflammatory signals within the recipient. This knowledge will guide the development of novel strategies to support Breg recovery and prevent or attenuate GVHD, such as infusion of donor-derived Bregs early in the patient’s posttransplant course.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health National Cancer Institute grants P01 CA148600-02 and RO1 CA061508-18. The flow studies were performed in the Flow Cytometry and Cellular Imaging Facility, which is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support grant CA016672.
Authorship
Contribution: A.S. performed experiments and designed, interpreted, analyzed, and wrote the manuscript; R.B. performed and analyzed experiments; R.S.M., H.S., M.M., T.S., A.K., E.G., K.K., D.M., A.M.A., A.A., E.L., M.D., B.O., A.O., R.B.J., U.P., C.H., R.C., and E.J.S. provided advice on experiments and commented on the manuscript; and K.R. designed and directed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katayoun Rezvani, Stem Cell Transplantation and Cellular Therapy, MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77004; e-mail: krezvani@mdanderson.org.



![Figure 6. B cells from patients undergoing CBT show an early and robust reconstitution of both the CD19+ B cells and the IL-10+ B-cell pools. (A) IL-10 secretion by total CD19+ B cells. PBMCs collected from 27 CBT patients and cultured with CD40L for 48 hours were stained for the CD19+IL-10+ phenotype by intracellular flow cytometric staining. The samples represented the following collection time points: pretransplant (n = 19), 1 month (n = 20), 3 months (n = 22), 6 months (n = 16), 9 months (n = 12), 1 year (n = 12) and 2 years (n = 8) post-CBT. Supernatants were also harvested from cultures of activated patient-derived B cells and assayed for IL-10 secretion by ELISA from samples collected at the following times: pretransplant (n = 12), 1 month (n = 10), 3 months (n = 10), 6 months (n = 10), 9 months (n = 8), 1 year (n = 12), and 2 years (n = 8) post-CBT. Bars indicate mean values and ranges (whiskers). (B) Absolute counts of IL-10+CD19+ B cells in PBMCs collected from 27 CB recipients, available at the following time points: pretransplant (n = 19), 1 month (n = 20), 3 months (n = 22), 6 months (n = 16), 9 months (n = 12), 1 year (n = 12) and CD19+B cells at 2 years (n = 8) post-CBT. (C) Comparison of IL-10 secretion by activated total CD19+ B cells in PB (n = 10) and CB (n = 10) from healthy donors and patients at 6 months posttransplant (n = 10). Bars denote median values and ranges (whiskers). *P < .05 by nonparametric ANOVA. (D) CD19+ B-cell frequencies and absolute counts per microliter in patients with cGVHD (n = 12) compared with patients without cGVHD (n = 15). *P < .05 and **P < .01 by unpaired t test. (E) CD19+IL-10+ B-cell frequencies and absolute counts per microliter in patients with cGVHD (n = 12) compared with patients without cGVHD (n = 15). *P < .05 and **P < .01 by unpaired t test. In both panels D and E, samples from patients with GVHD were available at the following time points: pretransplant (n = 9), 1 month (n = 10), 3 months (n = 10), 6 months (n = 7), 9 months (n = 6), 1 year (n = 5), and 2 years (n = 3) post-CBT. Samples from patients without GVHD were available at the following time points: pretransplant (n = 10), 1 month (n = 10), 3 months (n = 12), 6 months (n = 9), 9 months (n = 6), 1 year (n = 7), and 2 years (n = 5) post-CBT. The data points are mean values with ranges (whiskers). (F) Reconstituting IL-10–producing regulatory CD19+ B cells from patients possess greater suppressive function on T cells as compared with healthy CB B cells or PB B cells from healthy donors on a cell-per-cell basis. CD19+ B cells magnetically isolated from post-CBT patients at different time points post-CBT were cultured at a 1:1 ratio with anti-CD3/anti-CD28-activated CFSE+CD4+ T cells from healthy individuals for 96 hours, and then harvested and stained for CD4+CFSE+ proliferating T cells (healthy cord blood CD19+ B cells [n = 12]; CD19+ B cells at 1 month [n = 3]; CD19+ B cells at 3 months [n = 5]; CD19+ B cells at 6 months [n = 8]; CD19+ B cells at 9 months [n = 7]; CD19+ B cells at 1 year [n = 8]; CD19+ B cells at 2 years [n = 3]). (G) Patients with cGVHD (n = 6) had higher frequencies and absolute numbers of IFN-γ+CD4+ T cells compared with those without cGVHD (n = 4). (H) Patients with cGVHD (n = 6) had a reduced ratio of IL-10+ B cells to IFN-γ+CD4+ T cells compared with patients without cGVHD (n = 4). In both panels, bars denote mean values and ranges (whiskers). *P < .05 by unpaired t test. PBMC, peripheral blood mononuclear cell.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/10/10.1182_blood-2016-01-695122/4/m_1346f6.jpeg?Expires=1765890240&Signature=1zq9~06-7oNZQ5ykeUz~mZcVPTKTKqIuD0N9URdQxchIIguQBDGOvNWw4hxk1OMDYNTWEwt-2tDUQx8aOz-iCvIr1v0yoBSH0vFv~5DjqN41KnQVkQZzLnm24VEz~FC35TEZELjvHU953X0jAIpNHUw3PAocpBDTKjmHzRwzn7m~eaow9gJFfzXtG8DNrl4f7ijA0YeXkIs1D0j25BKWD6S~Jf3dK1wxROlrQnflAeZtpbeqiu8qc58mfYb4~HEF4VEwyNKOv1IYBpFTumMjmY0j5B6MrJs~ehQhxh5XfrZGb7LsOxgoWVi8E364NNjeHb-7waij-BGr5gHepQguCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal