In this issue of Blood, Khaw et al show that in contrast to the impressive antileukemic activity achieved by sole BCL-2 inhibition in chronic lymphocytic leukemia (CLL), optimal antileukemic activity in pediatric acute lymphoblastic leukemia (ALL) xenografts required concurrent inhibition of both BCL-2 and BCL-XL.1
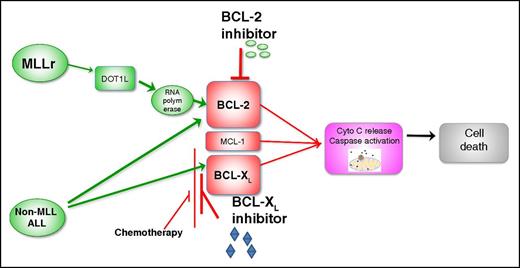
MLLr activates BCL-2 through H3K79 methylation rendering MLLr-ALL sensitive to selective BCL-2 inhibitor (venetoclax). In other subtypes of ALL, concurrent inhibition of both BCL-2 and BCL-XL is required for maximal antileukemia efficacy. Use of concurrent ALL chemotherapy that reduces MCL-1 and BCL-XL levels in combination with venetoclax may obviate the need for adding selective BCL-XL or dual BCL-2/BCL-XL inhibitors.
MLLr activates BCL-2 through H3K79 methylation rendering MLLr-ALL sensitive to selective BCL-2 inhibitor (venetoclax). In other subtypes of ALL, concurrent inhibition of both BCL-2 and BCL-XL is required for maximal antileukemia efficacy. Use of concurrent ALL chemotherapy that reduces MCL-1 and BCL-XL levels in combination with venetoclax may obviate the need for adding selective BCL-XL or dual BCL-2/BCL-XL inhibitors.
Venetoclax, a selective BCL-2 inhibitor, demonstrated inferior in vivo objective response of 26% as compared with an objective response of 61% with navitoclax, an inhibitor of BCL-2, BCL-XL, and BCL-W, in comparable xenograft panels of high-risk pediatric ALL.2 One important exception was the poor prognosis subgroup of pediatric mixed lineage leukemia-rearranged ALL (MLLr-ALL). Antagonism of BCL-2 alone proved efficacious in 50% of the MLLr-ALL xenografts as compared with 20% of non–MLLr-ALL xenografts. In vitro evaluation of navitoclax, venetoclax, or selective BCL-XL inhibitor (A-1155463) demonstrated that combined BCL-2 and BCL-XL inhibition by navitoclax was more potent than isolated inhibition of either pathway alone by venetoclax or by selective BCL-XL inhibitor A-1155463, respectively, across a broad range of B-cell ALL (B-ALL) and T-cell ALL (T-ALL) xenografts. There was a significant correlation between the responses of individual xenografts to navitoclax and venetoclax, but not A-1155463, suggesting that BCL-2 inhibition is of central importance, but on its own insufficient to induce maximal antileukemia activity in pediatric ALL.
Pediatric B-ALL is a heterogeneous disease with varying outcomes based on molecular subtype, age, white blood cell count at diagnosis, cytogenetics, day 14 bone marrow response, and post-induction minimal residual disease status. In the last decade, there has been significant progress in the therapy of patients with ALL with encouraging clinical activity demonstrated by monoclonal antibodies (mAbs) and chimeric antigen receptor (CAR) T cells. mAbs target highly expressed “leukemia” surface antigens and include (1) naked antibodies against common lymphoid markers such as anti-CD22 (epratuzumab), (2) antibody-drug conjugates linked to a highly potent toxin such as calicheamicin (inotuzumab ozogamicin), or (3) bispecific T-cell engaging agents that recruit and promote proximity induced cytotoxicity of tumor cells by T cells (blinatumomab).3,4 CAR T cells targeting CD19 have produced dramatic responses in heavily pretreated B-ALL patients. In spite of these breakthroughs, a fraction of children will be primary refractory or lose response to antigen-targeted immunologic therapies by target antigen downregulation or alternate bypass pathways. There remains a need for additional novel single-agent and combinatorial approaches in the therapy of ALL, especially in patients with high-risk or relapsed disease.
The BCL-2 family includes proapoptotic proteins (BAX, BAK, BIM, and PUMA) and antiapoptotic proteins (BCL-2, BCL-X, BCL-W, and MCL-1). Hematologic malignancies are critically dependent upon the antiapoptotic BCL-2 family proteins for survival. Mutations or dysregulation of the BCL-2 family proteins are associated with tumor initiation, disease progression, and chemotherapy resistance in leukemias, making them compelling targets for antitumor therapy. Inhibition of BCL-2 with venetoclax has shown unprecedented clinical responses in CLL,5 producing 75% response rates in patients with relapsed high-risk CLL, including patients with resistance to fludarabine and those with chromosome 17p deletions, and was recently US Food and Drug Administration–approved for 17p-deleted CLL.
The benefits of targeting BCL-2 family proteins and the ideal protein targets in ALL are not as well defined as in CLL. The proapoptotic gene BIM was shown to be upregulated in pediatric B-ALL patients with rapid early response, whereas antiapoptotic gene BCL-2 was upregulated in patients with slow early response.6 Early response to therapy is a powerful predictor of outcome in pediatric ALL. Another major determinant of clinical outcomes in pediatric ALL is response to prednisolone. High expression of prosurvival BCL-2 family protein MCL-1 by gene profiling was associated with in vivo and in vitro resistance to prednisolone in infant MLL-ALL. Bachmann et al demonstrated that glucocorticoid resistance in pediatric ALL xenografts was attributable to failure to upregulate BIM expression after steroid exposure.7 The role of BCL-XL antiapoptotic protein is well defined in T-ALL, where it governs the survival of more mature T-ALL cells (with a notable exception of the so-called early T-cell precursor ALL that is predominantly BCL-2 dependent). These data suggest that BCL-2 family proteins play a central role in ALL pathogenesis, resistance to standard chemotherapy, and outcomes.
The study by Khaw et al suggests that unlike CLL, the inhibition of BCL-2 alone may not produce significant and durable responses in pediatric ALL. One possible explanation for the differential sensitivity to isolated BCL-2 inhibition between these 2 lymphoid malignancies is that BCL-XL plays a more important role in early lymphoid development as compared with mature B lymphocytes. ALL is a disease of early B lymphocytes, whereas CLL originates from mature B lymphocytes. When comparing BCL-2 family messenger RNA expression for responders vs nonresponders in pediatric ALL xenografts, the only gene found to be significantly upregulated in nonresponders was BCL2L1 (encoding for BCL-XL/XS). In keeping with this finding, simultaneous targeting of BCL-2 and BCL-XL with venetoclax and A-1155463 induced synergistic killing across multiple ALL xenografts. The one exception identified, where targeting BCL-2 alone appeared to be sufficient, was MLLr-ALL. Previous studies have demonstrated high expression of BCL-2 in MLLr pediatric ALL8 and shown activity of the 1st generation BCL-2 antagonists in MLLr-ALL, supporting the findings by Khaw et al.8 It has been recently shown that MLL/AF4 specifically upregulates the BCL-2 gene but not other BCL-2 family members via DOT1L-mediated H3K79 methylation at the BCL-2 locus, and this may explain the higher sensitivity of MLLr-ALL xenografts to venetoclax.9 These data suggest that venetoclax alone may be the agent of choice in children with MLLr-ALL, whereas navitoclax may be more efficacious in non-MLL pediatric ALL (see figure). However, BCL-XL inhibition is associated with potentially severe on-target thrombocytopenia, limiting the clinical use of navitoclax in acute leukemias. To this end, alternative strategies could be exploited, such as the combined use of selective BCL-2 inhibitors with standard chemotherapeutic agents or mAbs, shown to be highly efficacious in preclinical studies. Notably, several commonly used chemotherapeutic agents were shown to affect the expression of MCL-1 (L-asparaginase and antimitotics) and of BCL-XL (L-asparaginase, dexamethasone, and vincristine).9,10 It is anticipated that combinations of venetoclax with established antileukemic agents has the potential to significantly improve the response rates, durability of response, and potentially overcome traditional high-risk features in pediatric ALL.
Conflict-of-interest disclosure: The authors declare no competing financial interests.