In this issue of Blood, Lu et al report on the generation and characterization of erythrocytes from a dematin knockout mouse and demonstrate the previously unrecognized centrality of this protein to the junctional protein complex–mediated maintenance of red blood cell (RBC) membrane integrity.1
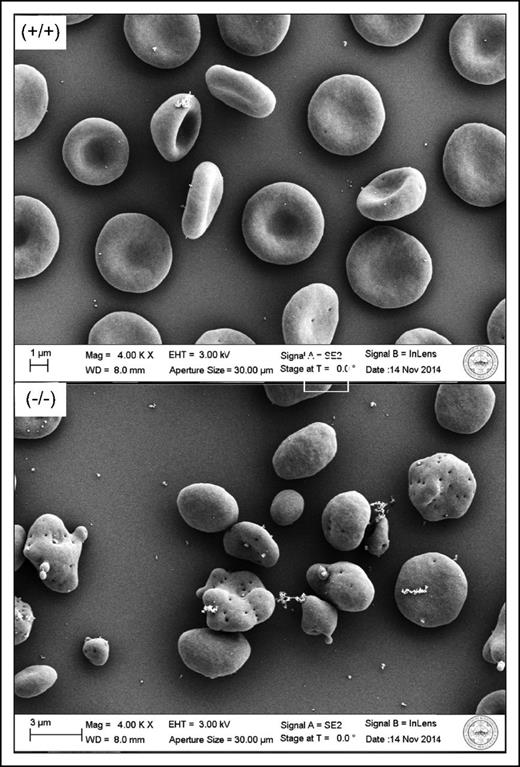
Electron microscope images of wild-type (+/+) and dematin knockout (−/−) erythrocytes, illustrating the membrane disturbance caused by the absence of dematin. The figure has been adapted from Figure 3D,H in the article by Lu et al that begins on page 93.
Electron microscope images of wild-type (+/+) and dematin knockout (−/−) erythrocytes, illustrating the membrane disturbance caused by the absence of dematin. The figure has been adapted from Figure 3D,H in the article by Lu et al that begins on page 93.
Ever since the first pioneering experiments using sodium dodecyl sulfate–polyacrylamide gel electrophoresis on RBC ghosts in the 1960s enabled the separation of the major abundant proteins that comprise the erythrocyte membrane, biologists have sought to uncover the details of the molecular organization of proteins that comprise this unique cell structure. The identification of 2 critical adaptor proteins, ankyrin and protein 4.1, which provide connectivity between the actin-linked spectrin tetramer-based membrane skeleton and the plasma membrane via interactions with transmembrane proteins band 3 and glycophorin C (GPC) provided the framework for our current understanding of erythrocyte membrane architecture and the basic “2 connectivity site model.” In the years since, many fine studies have expanded our knowledge of the additional protein components that reside at these 2 sites, giving rise to the concepts of the familiarly depicted “ankyrin-based” and “junctional” complexes that are vital for red cell membrane structure and function.2,3 Although evidence of the role of ankyrin in mediating vertical connectivity between spectrin and band 3 within the plasma membrane accumulated and is now well established, the concept that protein 4.1 via interaction with GPC alone performs this role within the junctional complex has remained relatively unchallenged in spite of the absent spherocytic phenotype one would expect associated with GPC deficiency.4
More recently, a role for adducin in mediating membrane cytoskeletal connectivity at the site of the junctional complex has been proposed based on an identified interaction of this protein with band 35 and the presentation of mild spherocytosis in α adducin knockout mice.6 In this issue, Lu and colleagues present data that establish a central role for the actin-binding protein dematin in the maintenance of erythrocyte membrane integrity. Through extensive characterization of the properties and membrane-cytoskeletal protein composition of RBCs isolated from a complete dematin knockout mouse, the authors identify secondary deficiencies of adducin, actin, and spectrin with a striking accompanying disruption in RBC membrane integrity (see figure) at least equivalent to that observed in ankyrin and band 3 null models. This work revises our current understanding of the hierarchy of protein interactions that exist within the junctional protein complex, positioning dematin as the hub and major determinant of membrane stability conferred by this site.
Surprisingly, in addition to the disruptive effects observed upon erythrocyte membrane structural integrity, dematin knockout fetal liver erythroblasts were found to exhibit enhanced or accelerated enucleation, not observed previously in alternative mouse models displaying gross disruptions of membrane integrity. Dematin performs an actin-binding and bundling function in erythroid cells and, in spite of significant progress in recent years, the precise mechanism underlying the process of enucleation (including the full role actin plays) remains to be determined. Elucidation of the mechanistic contribution of dematin as an apparent negative regulator of nuclear extrusion will undoubtedly be of interest to the field.
Clearly, a key question arising from this work is to what degree the findings observed here in mice can be extrapolated to the human context. In spite of many similarities, significant differences are notable in the membrane composition of mouse and human erythrocytes.7 The junctional complex in particular has been shown to contain Rh polypeptides in mice8 but not humans, whereas glucose transporter 1 (Glut1), which associates with dematin and the adducins in humans,9 is not expressed in mature murine erythrocytes. The authors propose a model in which adducin stabilized by dematin provides linkage to the plasma membrane via band 3, although the relatively mild phenotype of the α adducin knockout mouse6 argues for additional linkages likely via dematin directly. The search for and identification of such proteins within the mouse erythrocyte membrane will undoubtedly uncover new insights that will hopefully shed further light on our evolving understanding of membrane-cytoskeletal associations in the RBC. It will also be important to determine the role of dematin, and the effect of its deficiency in junctional complex assembly, regulation of membrane deformability and stability in human erythrocytes, and in the context of its identified association with Glut1. Given the importance of phosphorylation in regulation of dematin-binding function and interactions, and in light of the gross disruptive effects of dematin absence reported here, investigation of the role of dematin modification in junctional protein complex assembly, enucleation and cytoskeletal remodeling, and response to malaria invasion of the RBC will all represent important areas of future research.
Finally, as is often the case, where the “simple” RBC leads in terms of identifying new interactions and associations, the other cells of the body will follow. This new knockout mouse will be an exciting model system to explore the roles of dematin in platelets, the brain, and other tissues.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal