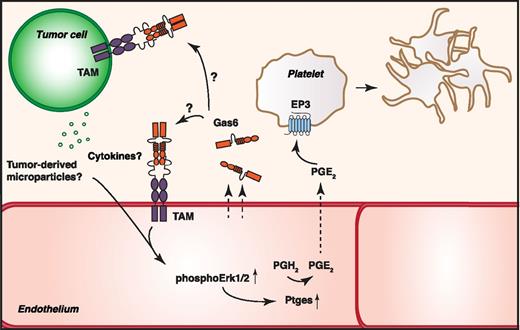
In this issue of Blood, Aghourian et al demonstrate that growth arrest-specific protein 6 (Gas6) contributes to tumor-induced venous thromboembolism (VTE) by promoting the expression of platelet-activating prostaglandin E2 (PGE2) in the endothelium.1
In the presence of endothelium-derived Gas6, tumor cells induce phosphorylation of Erk1/2 in endothelial cells, leading to an upregulation of Ptges production. Ptges produces PGE2 from prostaglandin H2 (PGH2), which, on secretion, interacts with the EP3 receptor on platelets to trigger platelet activation and promote thrombosis. Whether the effects mediated by Gas6 are due to an interaction of Gas6 with the tumor cell or through an autocrine signaling in the endothelial cell is still unknown.
In the presence of endothelium-derived Gas6, tumor cells induce phosphorylation of Erk1/2 in endothelial cells, leading to an upregulation of Ptges production. Ptges produces PGE2 from prostaglandin H2 (PGH2), which, on secretion, interacts with the EP3 receptor on platelets to trigger platelet activation and promote thrombosis. Whether the effects mediated by Gas6 are due to an interaction of Gas6 with the tumor cell or through an autocrine signaling in the endothelial cell is still unknown.
VTE is a well-recognized complication of different malignancies and substantially contributes to cancer-associated mortality. Understanding how different tumors modulate hemostasis is of the utmost importance for the development of next generation anticancer therapeutics, as well as for modulating current treatment regimes (see later in text). The occurrence of cancer-associated VTE is explained partially by the ability of tumors themselves to promote a procoagulant milieu both locally and systemically. For example, tumors may express tissue factor (TF) themselves or secrete procoagulant tissue factor-bearing microvesicles and release proinflammatory cytokines and proteases, resulting in activation of the coagulation cascade and downregulation of anticoagulant pathways.2
Aghourian et al unravel a novel link between tumor-mediated thrombosis and Gas6, which may provide yet another explanation for the procoagulant phenotype of many tumors. Gas6 is a vitamin K-dependent ligand for the receptor tyrosine kinase family comprising Tyro3, Axl, and Mer (TAM). It has the ability to bind to negatively charged phospholipids, such as phosphatidylserine exposed on apoptotic cells, which allows Gas6 to bridge TAM-expressing phagocytes to apoptotic cells thus potentiating phagocytosis. The Gas6-TAM system is a crucial anti-inflammatory signaling complex in immune cells, in addition to which it acts as a prosurvival factor, not only in tumors but also in the endothelium. Despite being homologous to the anticoagulant protein S, Gas6 has been shown to act mainly as a procoagulant factor. Gas6−/− mice are protected against lethal venous thrombosis, explained by a weakened platelet aggregation response3 and by decreased tissue factor expression in the endothelium.4 However, as the in vitro findings regarding platelet responses are of much milder character than what could be expected based on the striking phenotype of Gas6−/− mice, it is likely that yet unknown functions of Gas6 in regulation of thrombosis will emerge.
The work of Aghourian et al establishes a novel prothrombotic function of Gas6 independent of endothelial tissue factor regulation. In this study, the authors investigated changes in protein expression patterns on coculturing of endothelial cells from wild-type (WT) and Gas6−/− mice with M27 lung cell carcinoma. They identified the prostaglandin E synthase (Ptges) as a target protein upregulated in endothelium from WT mice but not in endothelium from Gas6−/− mice. Ptges is induced under proinflammatory stimuli, such as on exposure to cytokines, and the authors speculate that tumor-secreted cytokines or tumor-derived microvesicles might drive the increased endothelial Ptges. In a FeCl3-induced venous thrombosis model in either WT or Gas6−/− mice challenged with M27 cells or a B-cell lymphoma, the authors show that Gas6 deficiency protects the mice from a tumor-mediated increase in thrombus size. In Gas6-expressing endothelium, tumor cells were able to induce extracellular signal-regulated kinase (Erk)1/2 activation, resulting in downstream upregulation of Ptges and subsequent production and release of PGE2. They further validate the biological significance of the increased PGE2 production by showing that PGE2 is able to activate platelets, an effect inhibited by treating platelets with an EP3 receptor antagonist. As blocking the EP3 receptor in vivo in tumor-challenged mice reduced thrombus size on FeCl3 injection only in WT mice but not in Gas6−/− mice, the authors suggest that Gas6 has a direct role in PGE2-mediated thrombus formation (see figure).
On activation, platelets secrete polyphosphate normally stored in dense granules. Polyphosphates of different lengths have been shown to initiate the intrinsic coagulation pathway, the shorter chain polyphosphate derived from platelets mainly by activating factor XI and factor V and by antagonizing the TF pathway inhibitor.5 Hence, the platelet activation initially triggered by an increased PGE2 production may develop to be a self-feeding loop by directly promoting coagulation. A recent study reported that prostate cancer cells secrete long-chain polyphosphate with the ability to directly activate factor XII,6 establishing a novel link between cancer and coagulation. Whether polyphosphates are directly involved in the thrombotic responses observed by Aghourian et al remain to be elucidated.
In this study, Aghourian et al did not address the relative contribution of specific TAMs in mediating the Gas6-associated effects, nor did they analyze the expression of TAMs in murine endothelium. However, a recent publication from the same group demonstrates the presence of at least Axl in primary murine endothelial cells.7 TAMs, especially Axl, are in addition abundantly upregulated in several tumors, and it is likely that the tumor cells used in this study express at least one TAM member. Intriguingly, the question remains whether the Gas6 mediated prothrombotic effects are due to a direct autocrine Gas6 stimulation of endothelium or whether endothelium-derived Gas6 interacts with tumor-associated TAMs to modulate the secretion of thrombosis-promoting cytokines and/or microvesicles from the tumor. Endothelial cells from Gas6−/− mice have been shown to be less responsive to proinflammatory stimuli,8 and therefore it is possible that the endothelium-derived Gas6 might both affect the actual tumor cell and modulate endothelial responses to a tumor challenge.
Several therapeutic approaches to modulate TAM activity are under development, with the potential to be used as anti-inflammatory agents, vaccine adjuvants, and anticancer therapeutics. Upregulation of TAM expression has been shown to promote tumor survival and resistance to targeted therapies,9 and therefore TAM inhibition has been hypothesized to slow down or prevent tumor growth. With the findings of Aghourian et al, the therapeutic efficacy of TAM inhibitors extends to prevention of cancer-associated VTE, a highly desirable outcome with potential to affect survival rates. TAMs have several crucial functions throughout the body; consequently, therapeutic approaches should preferably target an individual TAM member to maintain TAM-mediated basal functions in other organs. Therefore, further studies exploring the relative contributions of the different TAMs as Gas6 receptors in the vasculature will be of high interest.
In conclusion, this elegant study provides further rationale to explore the function of Gas6 and its receptors in the aspect of both tumor pathogenesis and vascular biology and provides a novel concept of how Gas6 may promote tumor-associated adversities.
Conflict-of-interest disclosure: The authors declare no competing financial interests.