In this issue of Blood, Yang et al show the unexpected cooperation between enhancer of zeste homolog 2 (Ezh2) deletion and Jak2V617F, in switching the myeloproliferative neoplasm (MPN) phenotype in a mouse model. Their work also provides new therapeutic targets to block the progression of myelofibrosis (MF).1
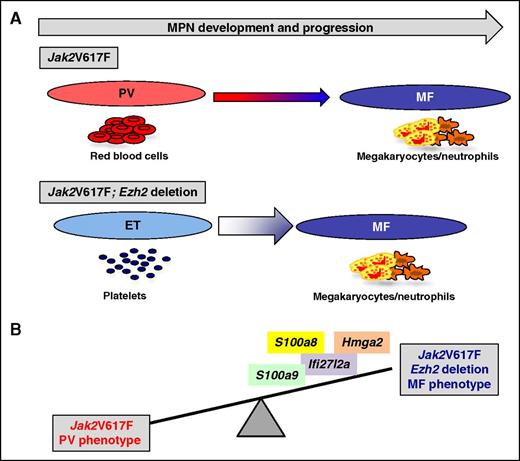
Ezh2 deletion has switched the MPN phenotype. (A) Jak2V617F knock-in mice develop a PV-like phenotype that progresses after 6 months into MF. Ezh2 deletion has switched Jak2V617F knock-in mice to an ET phenotype, quickly progressing to MF. (B) Ezh2 deletion in cooperation with Jak2V617F led to an increase in TNF-α, JNK, and IFN-α signaling, and overexpression of derepressed target genes such as S100a8, S100a9, Ifi27l2a, or Hmga2. Over-expression of these genes in Jak2V617F cells induces an MF phenotype.
Ezh2 deletion has switched the MPN phenotype. (A) Jak2V617F knock-in mice develop a PV-like phenotype that progresses after 6 months into MF. Ezh2 deletion has switched Jak2V617F knock-in mice to an ET phenotype, quickly progressing to MF. (B) Ezh2 deletion in cooperation with Jak2V617F led to an increase in TNF-α, JNK, and IFN-α signaling, and overexpression of derepressed target genes such as S100a8, S100a9, Ifi27l2a, or Hmga2. Over-expression of these genes in Jak2V617F cells induces an MF phenotype.
Non–BCR-ABL MPNs are clonal hematologic malignancies due to defects in hematopoietic stem cells (HSCs), leading to the amplification of one or more myeloid lineages. They include essential thrombocythemia (ET), polycythemia vera (PV), and primitive MF (PMF), characterized by the overproduction of platelets, red blood cells (RBCs), and the deregulation of the megakaryocytic/granulocytic lineages, respectively. They are due to acquired mutations in genes affecting the cytokine receptor/JAK2 signaling pathway. The main recurrent mutations involve JAK2V617F, the thrombopoietin receptor (MPL) gene, and the calreticulin gene.2 These are the “driver” mutations that are responsible for the disease phenotypes, as demonstrated by mouse modeling. Most Jak2V617F mice present a PV-like phenotype that progresses to MF after 6 months without leukemia development.3
In addition, these “driver” mutations are sometimes associated with others mutations, particularly in MF. They are mutations in the epigenetic regulators, components of the splicing machinery, or transcriptional factors. These associated mutations are not responsible for the myeloproliferation but are phenotypic modifiers of MPNs, either by favoring the clonal dominance of JAK2V617F cells (TET2 and DNMT3A mutations) by inducing myelodysplastic components (U2AF1 and SF3B1), or by favoring the progression to MF (ASXL1) or the transformation to secondary leukemia (IDH1/2, P53).2
EZH2 is a histone methyltransferase that cooperates with components of the polycomb repressive complex 2 to catalyze the trimethylation of lysine 27 of histone H3, in order to repress the transcription of target genes. Loss-of-function mutations of EZH2 are present in PMF (∼6% to 13%) and other myeloid malignancies, whereas gain-of-function mutations have been identified in B-cell malignancies. Ezh2 knockout mice die at day E7.5, demonstrating its essential role in development. Using a conditional Ezh2 deletion and tamoxifen-inducible, Cre-mediated recombination, a defect in lymphopoiesis, increased platelet count and decreased hemoglobin were observed.4,5 This model also confers a growth advantage to HSCs and mice develop a highly penetrant lethal MPN, myelodysplastic syndrome (MDS), or MPN/MDS, than can be associated with a high platelet count, extramedullar hematopoiesis, splenomegaly, anemia, and dysplasia of myeloid cells. In contrast, in the present study, the authors did not observe significant changes in hematopoiesis using a conditional Ezh2 deletion and interferon (IFN)-inducible Cre-mediated recombination. This discrepancy may be due to the experimental procedure and to the specific deletion of Ezh2 in different types of cells depending on the use of different conditional Cre-expression models.
Yang et al examined the cooperation between the deletion of Ezh2 and Jak2V617F in the development of MPN in a mouse model. As previously published by this group and others, the Jak2V617F knock-in mice develop a PV-like disease, which progresses to MF. Here, they observed that Ezh2 deletion in the presence of Jak2V617F severely impacted the phenotype of the MPN by inducing a great thrombocytosis, splenomegaly, and a rapid progression to MF. Strikingly, no PV-like phenotype was observed with normal values of hematocrit and RBCs. Thus, Ezh2 deletion has switched the PV phenotype of Jak2V617F knock-in mice to an ET phenotype, quickly progressing to MF (see figure panel A). This disease was also cell intrinsic because it was transplantable into secondary recipients with an even faster phenotype than primary recipients, leading to rapid death of the animals, that resembles what is observed with pure MPL activation in mouse models with either a gain-of-function mutation (MPLW515L) or the overexpression of thrombopoietin.2 Ezh2 deletion, like Tet2 deletion, has changed the fitness of Jak2V617F HSC by outcompeting HSC-expressing Jak2V617F alone. Thus, Ezh2 deletion cooperates with Jak2V617F and favors the clonal dominance at the HSC level. Moreover, the authors found higher numbers of megakaryocyte (MK) progenitors with an increase in the percentage of MKs in the bone marrow and spleen, whereas the erythroid progenitors were decreased with no growth of endogenous erythroid colony and with a blockade in the maturation of erythroblasts. Some factors have previously been identified as modifying the disease phenotype both in mouse models and clinical samples that could explain the phenotypic MPN heterogeneity. For instance, STAT1 is differentially activated in progenitors from JAK2V617F-ET vs -PV patients and overexpression of STAT1 in normal progenitors induces an increase in the number of MKs.6 Meanwhile, the Stat1 knockout mice showed decreased megakaryopoiesis and increased erythropoiesis.7 Moreover, when TET2 or DNMT3A mutation appears after JAK2V617F, the phenotype is mostly erythroid, whereas when they predate JAK2V617F, it gives a megakaryocytic advantage.8 Finally, germ line factors such as the HBS1L-MYB variant, which predisposes to ET by decreasing MYC expression, could also contribute to the megakaryocytic/erythroid equilibrium.9
Yang et al also focused on the mechanism of the cooperation using mainly transcriptomic and Chip-Seq analyses allowing the finding of bona fide targets of EZH2. Overall, they found that Ezh2 deletion led to an increase in tumor necrosis factor-α (TNF-α), JNK, and IFN-α signaling. The latter pathway may be a key determinant to consider because IFN-α activates STAT1, and STAT1 has been reported to be a central player in megakaryopoiesis by comparing ET vs PV patients. They also found that EZH2 controls the expression of transforming growth factor-β, which is the key cytokine responsible for the development of MF. Some of the genes derepressed by Ezh2 deletion (S100a8, S100a9, Ifi27l2a, or Hmga2) increased megakaryopoiesis when expressed in Jak2V617F cells (see figure panel B). Moreover, Ezh2-deletion–mediated overexpression of S100a8 and S100a9 could explain the inhibition of erythroid differentiation because they are responsible for the anemia in the Rps14-haploinsufficient mouse model of MDS.10
In conclusion, this study is a proof-of-concept that genes derepressed by EZH2 deletion should be targeted in patients displaying MF. Further investigation should help to confirm if heterozygous loss-of-function EZH2 mutations induce the same pattern of gene expression in patients. It remains to be determined if the same pathways are deregulated in PMF that are not associated with EZH2 mutations. If this is so, it would be worth developing molecules to modulate IFN-α signaling and thus STAT1/2 activation, and to inhibit JNK for the treatment of patients with PMF.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal