In this issue of Blood, Dietrich et al report that the low-dose BRAF inhibitor, vemurafenib, is highly effective in refractory hairy cell leukemia.1
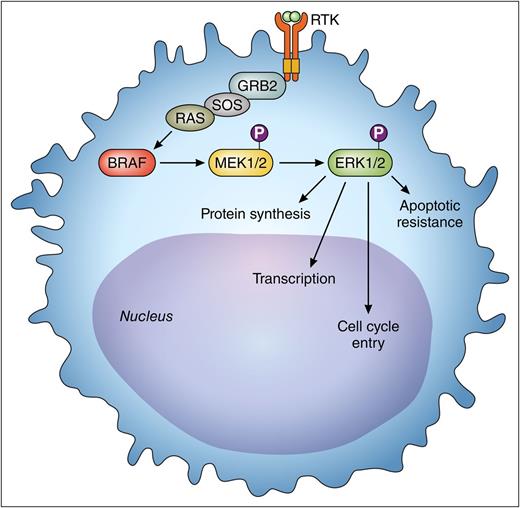
The activated BRAF pathway provides oncogenic signaling to the leukemic hairy cell through the MEK-ERK cascade. Vemurafenib is an inhibitor of BRAF in this pathway. The activity of pERK is reduced as a consequence of BRAF inhibition. The reduced pERK results in decreased cell proliferation. This is measured by immunohistochemical analysis and is used as a pharmacodynamics biomarker of BRAF inhibition. P, indicates a phospho-group; RTK, receptor tyrosine kinase. Professional illustration by Patrick Lane, ScEYEnce Studios.
The activated BRAF pathway provides oncogenic signaling to the leukemic hairy cell through the MEK-ERK cascade. Vemurafenib is an inhibitor of BRAF in this pathway. The activity of pERK is reduced as a consequence of BRAF inhibition. The reduced pERK results in decreased cell proliferation. This is measured by immunohistochemical analysis and is used as a pharmacodynamics biomarker of BRAF inhibition. P, indicates a phospho-group; RTK, receptor tyrosine kinase. Professional illustration by Patrick Lane, ScEYEnce Studios.
Despite enormous progress that has been made with purine nucleoside analogs as the initial treatment of patients with hairy cell leukemia, the relapse rate and eventual development of refractory disease mandate the continued search for effective new therapies. The discovery of the presence of the BRAF V600E mutation in the overwhelming majority of patients with classic hairy cell leukemia prompted the logical application of inhibitors of this oncogene for treating patients with relapsed or refractory disease.2 Extensive work had previously been done to define a dose and schedule of vemurafenib for the treatment of malignant melanoma. Encouraging results indicated that this agent produced responses in patients with melanoma, but relapse and resistance were frequently encountered.3,4 Likewise, studies using the same dose and schedule of vemurafenib in patients with hairy cell leukemia have recently shown that responses are observed, but unfortunately relapses are also routinely encountered.5 Simply extrapolating the dose and schedule of this agent based on treatment plans established for patients with malignant melanoma underestimates the need to design specific therapeutic intervention based on the biology of the target in the patient with leukemia.
The current report provides important information on the dose-response relationship of vemurafenib in patients with refractory hairy cell leukemia, showing that the response rate and kinetics of response are independent of dosing. Furthermore, Dietrich et al1 show that abrogation of phosphorylation of extracellular signal-related kinase (ERK) as a downstream target was consistently observed with low-dose administration. Identification of a genomic target in patients with hairy cell leukemia with a potential pharmacodynamics end point enables a rational approach to optimizing treatment. In the figure, the BRAFV600E pathway results in the expression of phospho-ERK (pERK). In recurrent or resistant melanoma, it is postulated that the reexpression of pERK and abnormalities in the MAPK pathway may be involved in the pathogenesis of progressive disease.6 This pharmacodynamics end point will need to be further validated in other studies of hairy cell leukemia. Investigation of this oncogenic pathway will hopefully provide insight into new therapeutic strategies for treatment. Alternatively, other modalities may be incorporated into combination approaches to treat the residual leukemia (eg, anti-CD20 monoclonal antibodies; immunotoxin conjugates; other immunotherapeutic approaches; and novel targeted agents like Bruton tyrosine kinase inhibitors).
Dietrich et al1 accumulated important data by careful analysis of patients treated with this agent at variable doses. The clinical results are very similar to the published data using the higher dose routinely used in treating melanoma.5 The current study emphasizes that this agent will be highly useful in treating patients and suggests that additional work should be done to accurately define an optimal dose and schedule for administration. This further refinement is important because ultimate long-term control of this disease will likely involve strategic additions of other agents to circumvent the inevitable development of relapse and resistant disease. Fully defining the mechanisms of drug resistance will be a high priority for this disease. Although the overall survival for these patients has markedly improved, the long-term course is complicated by relapses requiring additional therapy.
Another potential important opportunity for using this agent involves managing patients with hairy cell leukemia complicated by an active infection.7 Vemurafenib toxicities include arthralgias, photoxicity, skin tumors, and some less common toxicities. However, this agent is not very myelosuppressive. The administration of vemurafenib has improved peripheral blood counts and has been used successfully in a neutropenic patient with an active infection.8 Infection remains one of the most serious complications of this disease.9 Patients who present with severe granulocytopenia and monocytopenia are subject to life-threatening infection. The myelosuppression and immunosuppression associated with the purine nucleoside analogs may be profound and prolonged, and several investigators have introduced vemurafenib to patients who needed treatment of the underlying leukemia in the face of infection. The limited initial treatment with this agent has enabled recovery of granulocytes and may lead to successful control of infection. The consolidation of the therapy for the underlying leukemia could be safely approached with standard therapy after the danger associated with the infection has been resolved. Dietrich et al suggest that this agent may have utility in the setting of infection and thus may be incorporated into new strategies for treatment of leukemia after the optimal dose and schedule of administration are ultimately determined.7
Optimizing therapy with targeted inhibitors requires detailed study of the kinetics of enzyme inhibition. In addition to adequate dosing, selection of patients with the genomic profile for the targeted therapy is an evolving goal in personalized medicine. Using pharmacodynamics end points provides a strategy to achieve the desired on-target antitumor activity and lessen off-target side effects. Design of these studies is challenging in a relatively rare disease. Although this disease is often considered rare, there are an increasing number of patients who now live longer and will encounter difficulties with multiple relapses requiring therapy. While studies designed to find the optimal dose and schedule of BRAF inhibitor administration alone are important, other studies to explore strategic combination therapies are also needed. Consequently, patients who have this diagnosis should be encouraged to participate in well-designed ongoing clinical trials. Despite the substantial progress in managing this leukemia, the disease has not been cured and requires continued organized clinical investigation.
Conflict-of-interest disclosure: M.R.G. is a nonpaid member of the Scientific Board for the Hairy Cell Leukemia Foundation.