Key Points
CAP-PEs, a novel type of oxidatively modified phospholipids, are present in vivo.
CAP-PEs can activate platelets via TLRs by inducing a cross-talk between innate immunity and integrin activation signaling pathways.
Abstract
A prothrombotic state and increased platelet reactivity are common in dyslipidemia and oxidative stress. Lipid peroxidation, a major consequence of oxidative stress, generates highly reactive products, including hydroxy-ω-oxoalkenoic acids that modify autologous proteins generating biologically active derivatives. Phosphatidylethanolamine, the second most abundant eukaryotic phospholipid, can also be modified by hydroxy-ω-oxoalkenoic acids. However, the conditions leading to accumulation of such derivatives in circulation and their biological activities remain poorly understood. We now show that carboxyalkylpyrrole-phosphatidylethanolamine derivatives (CAP-PEs) are present in the plasma of hyperlipidemic ApoE−/− mice. CAP-PEs directly bind to TLR2 and induces platelet integrin αIIbβ3 activation and P-selectin expression in a Toll-like receptor 2 (TLR2)-dependent manner. Platelet activation by CAP-PEs includes assembly of TLR2/TLR1 receptor complex, induction of downstream signaling via MyD88/TIRAP, phosphorylation of IRAK4, and subsequent activation of tumor necrosis factor receptor–associated factor 6. This in turn activates the Src family kinases, spleen tyrosine kinase and PLCγ2, and platelet integrins. Murine intravital thrombosis studies demonstrated that CAP-PEs accelerate thrombosis in TLR2-dependent manner and that TLR2 contributes to accelerate thrombosis in mice in the settings of hyperlipidemia. Our study identified the novel end-products of lipid peroxidation, accumulating in circulation in hyperlipidemia and inducing platelet activation by promoting cross-talk between innate immunity and integrin activation signaling pathways.
Introduction
Products of lipid peroxidation have been shown to accumulate in vivo in various pathological states, such as in atherosclerotic lesions and plasma of patients with coronary artery disease.1-4 Lipids containing polyunsaturated fatty acids are the major targets of free radical–initiated peroxidation. One consequence of lipid peroxidation of polyunsaturated fatty acids is the generation of a number of highly reactive aldehydes, which are capable of covalent modification of autologous proteins reacting with the lysine amino group, cysteine thiol group, or histidine imidazole group.5 Products of this reaction can be biologically active, and carboxyalkylpyrrole-protein derivatives (CAP proteins) are one example of such derivatives. CAP proteins are formed as a result of a reaction of hydroxy-ω-oxoalkenoic acids with primary amino groups of protein lysines.6 CAP proteins have been recently found in vivo and have gained significant attention because of their role in vascular endothelial growth factor receptor–independent angiogenesis, wound healing, and platelet activation.7,8 CAP proteins were shown to induce cellular responses via Toll-like receptors (TLRs). Phosphatidylethanolamine (PE), the second most abundant phospholipid in mammalian membrane, possesses a primary amino group, and could be a target for modification by reactive aldehydes generated during lipid peroxidation.9 Indeed, recent in vitro studies have shown that PE is the preferable target for modification by some lipid aldehydes.10 Carboxyalkylpyrrole-PE derivatives (CAP-PEs) were recently detected in human blood.11 However, whether CAP-PEs accumulate in circulation in dyslipidemia, whether they can affect the function of platelets and thrombosis, and what the receptors and signaling pathways in platelets implicated by the novel derivatives is not known.
TLRs, receptors of innate immunity, are known to recognize pathogen-associated molecular patterns. Recent studies have demonstrated that a number of endogenous ligands, such as altered self-ligands, could also be recognized by TLRs12-14 and contribute to progression of inflammation and atherosclerosis. As the role of platelet TLRs in response to pathological ligands in the circulation has started to emerge,8,14-16 whether endogenous ligands regulate thrombosis by engaging TLRs on platelets and the specific signaling pathways activation have not been known. In particular, the pathways connecting innate immunity and platelet integrin activation are not known.
In this study, we demonstrated CAP-PEs are present in vivo in circulation in conditions of hyperlipidemia. CAP-PEs can activate both the human and murine platelets by inducing an assembly of TLR2/TLR1 complex on platelets, activating an innate immunity signaling cascade, and establishing a cross-talk with platelet activation signaling cascade via tumor necrosis factor receptor–associated factor 6 (TRAF6) and Src family kinases (SFKs).
Methods
Additional methods are presented in the supplemental Methods, available on the Blood Web site.
Preparation of platelets
Flow cytometry
Human and murine platelet suspensions were prepared by gel filtration, as described previously,8 and incubated with various blocking reagents and agonists indicated in the figure legends. P-selectin expression and integrin αIIbβ3 activation were assessed as described previously.8,16 Data were acquired using a fluorescence-activated cell sorter (FACS) Calibur instrument (Becton Dickinson, San Jose, CA) and analyzed using FlowJo 9.4 software (Tree Star, Ashland, OR).
Platelet aggregation
Platelet aggregation was monitored using a Chronolog Model 560VS aggregometer with AGGRRO/LINK, version 5.1.9, software, at a stirring speed of 1000 rpm. Aliquots (200 μL) of PRP or aliquots (500 μL) of gel-filtered platelets were placed in cuvettes containing magnetic stirrer bars, warmed at 37°C, and stirred for 90 seconds to obtain a stable baseline. Platelet concentration was adjusted to 2 × 108 platelets/mL using either platelet poor plasma of the same genotype or buffer for gel-filtered platelets.
Coimmunoprecipitation
Platelets isolated by gel filtration were stimulated with agonist in Tyrode’s buffer (Ca2+, Mg2+) at 37°C for 15 minutes. Platelets were then pelleted down and lysed with radioimmunoprecipitation assay buffer on ice. Protein concentrations were estimated and equal amount of total protein was taken and first precleared with immunoglobulin G (IgG) and then incubated with antibody overnight at 4°C with gentle rotation. Protein A/G plus-agarose beads were then added and incubated for 2 to 4 hours at 4°C with gentle rotation. Beads were then washed 3 times, boiled with Laemmli protein sample buffer, and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analysis.
Intravital thrombosis
Intravital thrombosis study was performed using FeCl3-induced carotid artery thrombosis in hyperlipidemic and normolipidemic mice of indicated genotype, as previously described.8 Where indicated, wild-type (WT) and TLR2−/− mice received 20 μM carboxyethylpyrrole (CEP-PE) or PE (control) in phosphate-buffered saline via tail vein injection. The blood vessels were observed under a Leica DM LFS microscope (Leica, Germany) with ×10/0.30 objectives. Images were acquired using a cooled high-speed, digital camera (QImaging Retiga EXi Fast 1394) and with Streampix acquisition software.
Statistical analysis
Data are presented as mean ± standard error of the mean. The statistical significance of differences was evaluated using the 2-tailed nonparametric Mann-Whitney U test for in vivo thrombosis experiments and Student t test for others. Values of P < .05 were considered as statistically significant.
Results
CAP-PEs induce platelet activation
CEP-PE), carboxypropylpyrrole (CPP-PE), and carboxyheptylpyrrole (CHP-PE) derivatives of phosphatidylethanolamine are generated as a result of oxidation of the 3 most abundant plasma polyunsaturated fatty acids (docosahexaenoic, arachidonic, and linoleic acids) (supplemental Figure 3A). They were synthesized as described11 (supplemental Figures 1 and 2) and tested in several platelet activation assays. CAP-PEs induced significant P-selectin expression (Figure 1A-B) and integrin αIIbβ3 activation (Figure 1C-D) in isolated human platelets. CAP-PEs induced platelet activation in a concentration dependent manner with significant effect observed at submicromolar concentrations (supplemental Figure 3B-C). Similarly, CAP-PEs induced activation of isolated murine platelets (Figure 1E-F). Unmodified PE had no effect on platelets.
CAP-PEs induce platelet activation. (A-D) Human platelets were isolated by gel filtration and were incubated with indicated agonists (A,C) CEP-PE (10 μM), PE (10 μM), ADP (10 μM), thrombin (Thr; 0.05 U/mL), and (B,D) CHP-PE (10 μM). Then, P-selectin (A-B) expression or integrin αIIbβ3 (C-D) activation was assessed by FACS analysis. (E, F) Murine platelets were isolated by gel filtration and incubated with (E) CEP-PE (20 μM), (F) CHP-PE (20 μM), or control agonists including PE (20 μM), ADP (10 μM), or thrombin (0.05 U/mL). Integrin αIIbβ3 activation was assessed by FACS analysis. (Left) Histogram presentations of FACS analysis data; (right) quantification data (means ± SD) from 3 independent experiments. ***P < .001 vs resting platelets.
CAP-PEs induce platelet activation. (A-D) Human platelets were isolated by gel filtration and were incubated with indicated agonists (A,C) CEP-PE (10 μM), PE (10 μM), ADP (10 μM), thrombin (Thr; 0.05 U/mL), and (B,D) CHP-PE (10 μM). Then, P-selectin (A-B) expression or integrin αIIbβ3 (C-D) activation was assessed by FACS analysis. (E, F) Murine platelets were isolated by gel filtration and incubated with (E) CEP-PE (20 μM), (F) CHP-PE (20 μM), or control agonists including PE (20 μM), ADP (10 μM), or thrombin (0.05 U/mL). Integrin αIIbβ3 activation was assessed by FACS analysis. (Left) Histogram presentations of FACS analysis data; (right) quantification data (means ± SD) from 3 independent experiments. ***P < .001 vs resting platelets.
Class B scavenger receptors CD36 and SR-BI are not involved in platelet responses to CAP-PEs
Class B scavenger receptors CD36 and SR-BI are expressed on platelets and recognize oxidized phospholipids.17,18 To assess whether these receptors play a role in the effects of CAP-PEs on platelets, we first tested the effect of Fab fragments of anti-CD36 and anti–SR-BI blocking antibodies on CAP-PE–induced human platelet activation. We found no significant effect of either of the blocking antibodies (supplemental Figure 4A-B). Furthermore, murine platelets isolated from CD36−/− (supplemental Figure 4C) or SR-BI−/− (supplemental Figure 4D) mice were activated by CAP-PEs similar to WT mice demonstrating that the activation of platelets by CAP-PEs is not mediated by CD36 or SR-BI.
CAP-PEs are recognized by TLR2 and activate platelets via TLR2/TLR1
TLRs 1-9 are expressed in platelets and are known to recognize foreign lipid and lipoproteins.8,12,13,19-21 The TLR2 blocking antibody strongly inhibited platelet response to CAP-PEs, whereas nonimmune isotype matched IgG (Figure 2A; supplemental Figure 5A) and TLR3-, TLR4-, and TLR9-specific blocking antibodies had no effect (supplemental Figure 5D-F). TLR2 functions as a heterodimer either with TLR1 or TLR6. We observed significant inhibition of platelet activation by the TLR1 blocking antibody (Figure 2B; supplemental Figure 5B), but not by the TLR6 blocking antibody (supplemental Figure 5C), suggesting that TLR1 serves as a coreceptor for TLR2 and mediates CAP-PE–induced platelet activation. To further demonstrate the involvement of TLR2 in CAP-PE–induced platelet activation, we performed a platelet activation experiment using murine platelets isolated from TLR2−/−, TLR6−/−, TLR9−/−, and WT mice. We observed a significant reduction of platelet activation by CAP-PEs only in TLR2−/− mice (Figure 2C; supplemental Figure 5G-H).
CAP-PEs are recognized by TLR2 and activate platelets via TLR2/TLR1. (A-B) Human platelets were isolated by gel filtration and preincubated with either blocking antibody (for TLR2 and TLR1) or nonimmune isotype-matched control IgG and then stimulated with CAP-PEs (CEP-PE). P-selectin expression was assessed by FACS analysis. (Right) Quantification of the data. (C) Murine platelets were isolated from WT and TLR2−/− mice by gel filtration, stimulated by 20 μM CAP-PEs (CHP-PE or CEP-PE) and integrin αIIbβ3 activation was assessed by FACS analysis. (D) CEP-PE, CHP-PE, or PE binding to TLR2 in the presence of increasing concentrations of CAP-PEs was determined by competitive enzyme-linked immunosorbent assay as described in the “Methods” section. (E) HEK-Blue-TLR2 and HEK-Blue-Null cells were incubated with 20 μM CAP-PEs (CEP-PE or CHP-PE) and TLR2 agonists Pam3CSK4 (25 ng/mL) or LTA (5 μg/mL) or control buffer (NT) for 16 to 18 hours. (F) HEK-Blue-TLR9 and HEK-Blue-Null cells were incubated with CAP-PEs (CEP-PE) and positive control CpG ODN2006 (100 nM). (G) HEK-Blue-TLR2 cells were preincubated with TLR2- or TLR1-blocking antibody or both the blocking antibodies, or IgG control for 12 hours, then incubated with the agonists for 16 to 18 hours. ***P < .001, **P < .01, comparing IgG vs blocking antibody or WT vs TLR2−/− or treated with NT.
CAP-PEs are recognized by TLR2 and activate platelets via TLR2/TLR1. (A-B) Human platelets were isolated by gel filtration and preincubated with either blocking antibody (for TLR2 and TLR1) or nonimmune isotype-matched control IgG and then stimulated with CAP-PEs (CEP-PE). P-selectin expression was assessed by FACS analysis. (Right) Quantification of the data. (C) Murine platelets were isolated from WT and TLR2−/− mice by gel filtration, stimulated by 20 μM CAP-PEs (CHP-PE or CEP-PE) and integrin αIIbβ3 activation was assessed by FACS analysis. (D) CEP-PE, CHP-PE, or PE binding to TLR2 in the presence of increasing concentrations of CAP-PEs was determined by competitive enzyme-linked immunosorbent assay as described in the “Methods” section. (E) HEK-Blue-TLR2 and HEK-Blue-Null cells were incubated with 20 μM CAP-PEs (CEP-PE or CHP-PE) and TLR2 agonists Pam3CSK4 (25 ng/mL) or LTA (5 μg/mL) or control buffer (NT) for 16 to 18 hours. (F) HEK-Blue-TLR9 and HEK-Blue-Null cells were incubated with CAP-PEs (CEP-PE) and positive control CpG ODN2006 (100 nM). (G) HEK-Blue-TLR2 cells were preincubated with TLR2- or TLR1-blocking antibody or both the blocking antibodies, or IgG control for 12 hours, then incubated with the agonists for 16 to 18 hours. ***P < .001, **P < .01, comparing IgG vs blocking antibody or WT vs TLR2−/− or treated with NT.
To test whether CAP-PEs directly bind to TLR2, we used a competitive enzyme-linked immunosorbent assay. CAP-PEs, but not unmodified PE, inhibited binding of soluble TLR2 to its ligand in a concentration-dependent manner (Figure 2D), suggesting CAP-PEs are the ligands for TLR2. Next, we tested the effect of CAP-PEs in alternative system HEK-Blue-TLR2 cells overexpressing hTLR2 and reporter gene under control of NF-κB. We observed a strong induction of reporter gene activity in response to CAP-PEs, comparable to these of established TLR2 agonists lipoteichoic acid (LTA) and Pam3CSK4 (Figure 2E). Control null cells showed no response. As an additional control, we incubated TLR9 overexpressing HEK-Blue-TLR9 cells with CAP-PEs or CpG ODN2006 as a positive control. CAP-PEs had no effect on TLR9 overexpressing cells, whereas positive control CpG ODN2006, as anticipated, induced strong response (Figure 2F). This experiment further demonstrates that activation of cells by CAP-PEs is specific to TLR2.
We observed a significant but incomplete inhibition of cell response to CAP-PEs by the TLR2 blocking antibody (Figure 2G). The anti-TLR1 antibody also had a strong but incomplete inhibitory effect (Figure 2G). A combination of anti-TLR1 and anti-TLR2 antibodies almost completely suppressed cell response to CAP-PEs (Figure 2G). This experiment further suggests that both TLR1 and TLR2 are involved in the activation of cells by CAP-PEs. Western blot analysis revealed the presence of a low level of TLR1 in the HEK-Blue-TLR2 cells (supplemental Figure 5I). Taken together, these experiments suggest that CAP-PEs act via TLR1 and TLR2 hetero-dimer.
CAP-PEs induce platelet aggregation in vitro and promote thrombosis in vivo in a TLR2-dependent manner
We next tested whether CAP-PEs induce platelets aggregation. We observed that CAP-PEs accelerated aggregation in gel-filtered human platelets (Figure 3A). In PRP, CAP-PEs alone did not induce platelet aggregation at concentrations used, likely because of sequestration into lipoproteins (very low-density lipoprotein, low-density lipoprotein, high-density lipoprotein) that are present in PRP. However, it promoted platelet hyperreactivity as evidenced by the accelerated and irreversible aggregation in response to a low dose of the physiological agonist adenosine 5′-diphosphate (ADP) (Figure 3B). This effect was TLR2-dependent because it was not observed in platelets of TLR2−/− mice (Figure 3C). Next, to test whether the presence of CAP-PEs in the circulation in mice accelerates thrombosis, we performed a FeCl3-induced carotid artery thrombosis assay in WT and TLR2−/− mice, which received IV injections of either CAP-PEs or control PE. CAP-PEs injection significantly shortened the occlusion time in the WT mice, but not in TLR2-deficient mice (Figure 3D).
CAP-PEs induce platelet aggregation and hyperreactivity in vitro and promote thrombosis in vivo in a TLR2-dependent manner. (A) Human platelets were isolated by gel filtration. A total of 2 × 108/mL platelets were stimulated either with 1 U/mL thrombin, or 20 μM CAP-PEs (CEP-PE or CHP-PE) in the presence of 1 mg/mL fibrinogen and platelet aggregation was monitored in a Lumi-Aggregometer. (B-C) Platelet-rich plasma, isolated from WT (B) or TLR2−/− (C) mice was primed with/without 20 μM CAP-PEs (CHP-PE), then stimulated with ADP and aggregation was monitored. (D) WT and TLR2−/− mice were injected with 20 μM CAP-PEs (CEP-PE) or PE control via tail vein and after FeCl3 injury; the time to complete thrombotic occlusion of carotid artery was recorded. (E) Presence of CAP-PEs (CEP-PE) in the plasma of ApoE−/− mice fed chow diet (CD) or Western diet (WD) was determined by Far-Eastern blotting as described in the “Methods” section. (Right) Plasma concentrations of CEP-PE assessed by densitometry of Far-Eastern blotting using synthetic CEP-PE as a standard. (F) ApoE−/−, ApoE−/−/CD36−/−, and ApoE−/−/CD36−/−/TLR2−/− mice fed either CD or WD were used in intravital thrombosis assay. After FeCl3 injury, the time to complete thrombotic occlusion of carotid artery was recorded. *P < .05, **P < .01, ***P < .001 and not significant, P > .05.
CAP-PEs induce platelet aggregation and hyperreactivity in vitro and promote thrombosis in vivo in a TLR2-dependent manner. (A) Human platelets were isolated by gel filtration. A total of 2 × 108/mL platelets were stimulated either with 1 U/mL thrombin, or 20 μM CAP-PEs (CEP-PE or CHP-PE) in the presence of 1 mg/mL fibrinogen and platelet aggregation was monitored in a Lumi-Aggregometer. (B-C) Platelet-rich plasma, isolated from WT (B) or TLR2−/− (C) mice was primed with/without 20 μM CAP-PEs (CHP-PE), then stimulated with ADP and aggregation was monitored. (D) WT and TLR2−/− mice were injected with 20 μM CAP-PEs (CEP-PE) or PE control via tail vein and after FeCl3 injury; the time to complete thrombotic occlusion of carotid artery was recorded. (E) Presence of CAP-PEs (CEP-PE) in the plasma of ApoE−/− mice fed chow diet (CD) or Western diet (WD) was determined by Far-Eastern blotting as described in the “Methods” section. (Right) Plasma concentrations of CEP-PE assessed by densitometry of Far-Eastern blotting using synthetic CEP-PE as a standard. (F) ApoE−/−, ApoE−/−/CD36−/−, and ApoE−/−/CD36−/−/TLR2−/− mice fed either CD or WD were used in intravital thrombosis assay. After FeCl3 injury, the time to complete thrombotic occlusion of carotid artery was recorded. *P < .05, **P < .01, ***P < .001 and not significant, P > .05.
To test whether CAP-PEs levels are increased in hyperlipidemia, a condition associated with oxidative stress, platelet hyperreactivity, and accelerated thrombosis, we isolated lipids from the plasma of ApoE−/− mice fed either a Western diet or a chow diet and assessed the levels of CAP-PEs using specific poly- and monoclonal antibodies and Far-Eastern blotting. CAP-PEs’ immunoreactivity was highly elevated in PE fraction of hyperlipidemic murine plasma as compared with plasma of mice fed a chow diet and reached 20 to 30 μM (Figure 3E; supplemental Figure 6A). High specificity of polyclonal and monoclonal antibodies was confirmed in a series of assays (supplemental Figure 6A-D). Furthermore, the presence of CEP-PEs in plasma of hyperlipidemic ApoE−/− mice was demonstrated using liquid chromatography tandem mass spectometry analysis (supplemental Figure 6H). Taken together, these data demonstrate that specific ligands for TLR2 accumulate in the circulation in hyperlipidemia and may promote thrombosis in a TLR2-dependent manner. To test whether TLR2 deficiency may confer protection from thrombosis in hyperlipidemia, we used ApoE−/−/CD36−/− mice. These mice were chosen because their platelets are protected from hyperreactivity induced by oxidized phospholipids ligands for CD36. We crossed ApoE−/−/CD36−/− mice to ApoE−/−/TLR2−/− mice and generated ApoE−/−/CD36−/−/TLR2−/− mice. Intravital thrombosis studies demonstrated a statistically significant increase in occlusion time in hyperlipidemic ApoE−/−/CD36−/−/TLR2−/− mice as compared with hyperlipidemic ApoE−/−/CD36−/− mice (Figure 3F; supplemental Figure 6F), supporting a role for TLR2 in prothrombotic phenotype associated with dyslipidemia. We observed slight increase of CEP-PE levels in double- and triple-knockout mice as compared with control mice (supplemental Figure 6E). This observation indicates that the thromboprotective phenotype in triple-knockout mice cannot be explained by the reduced levels of CAP-PEs. Importantly, we observed no significant difference in rate of thrombosis between WT and TLR2−/− mice fed a chow diet, demonstrating that TLR2 normally does not contribute to thrombosis (data not shown). Taken together, these data demonstrate that specific ligands for TLR2 accumulate in circulation in hyperlipidemia and may promote thrombosis in a TLR2/TLR1-dependent and a scavenger receptor CD36-independent manner.
TIRAP and MyD88 are involved in platelet activation by CAP-PEs
Myeloid-differentiation factor 88 (MyD88) is the universal adaptor for TLR signaling.22 TIRAP, another adaptor protein in the TLR pathway, facilitates the association of TLR2–TLR1 complex with MyD88.22 The TIRAP blocking peptide completely abolished CAP-PE–induced platelet P-selectin expression, whereas no effect of the control peptide was observed (Figure 4A), demonstrating that TIRAP is involved in the signaling pathway induced by CAP-PEs in platelets. The MyD88 blocking peptide also significantly inhibited platelet activation by CAP-PEs, whereas the control peptide had no effect (Figure 4B). A similar effect of the MyD88 blocking peptide was observed in HEK-Blue-TLR2 cells (Figure 4D), and the presence of MyD88 in these cells is shown by western blot analysis (Figure 4E). We also observed a significant reduction of CAP-PEs induced activation in platelets isolated from MyD88−/− mice as compared with platelets of WT mice (Figure 4C).
TIRAP and MyD88 are involved in platelet activation by CAP-PEs. (A-B) Gel-filtered human platelets were preincubated with (A) 10 μM TIRAP blocking peptide (TIRAP BP), (B) 10 μM MyD88 blocking peptide (MyD88 BP), or control peptide (CP), followed by stimulation with CAP-PEs (CHP-PE). (Left) Histogram presentation of FACS analysis data; (right) quantified data (mean ± SD) from 3 independent experiments. (C) Murine platelets were isolated by gel filtration from WT and MyD88−/− mice and were stimulated with CAP-PEs (CHP-PE); integrin αIIbβ3 activation was assessed by FACS analysis. (D) HEK-Blue-TLR2 cells were preincubated with MyD88 BP and CP for 12 hours and then stimulated with CAP-PEs (CEP-PE or CHP-PE) for 16 to 18 hours. (E) A total of 40 μg of total protein from TLR2-overexpressing cells and control cells (Null) were separated by SDS-PAGE and immunoblotted against MyD88 antibody. (F,H) Gel-filtered human platelets were stimulated with CAP-PEs (CEP-PE or CHP-PE), lysed, and (F-G) 300 μg of lysate protein was immunoprecipitated (IP) with (F) TLR2 antibody or (G) MyD88 antibody and immunoblotted against (F) TLR1 or (G) TLR2; (H) 50 μg of total protein was separated by SDS-PAGE and TLR2, and MyD88 and actin were detected by western blotting. ***P < .001, **P < .01, *P < .05, comparing either blocking vs control peptide or WT vs knockout mice or control vs treated.
TIRAP and MyD88 are involved in platelet activation by CAP-PEs. (A-B) Gel-filtered human platelets were preincubated with (A) 10 μM TIRAP blocking peptide (TIRAP BP), (B) 10 μM MyD88 blocking peptide (MyD88 BP), or control peptide (CP), followed by stimulation with CAP-PEs (CHP-PE). (Left) Histogram presentation of FACS analysis data; (right) quantified data (mean ± SD) from 3 independent experiments. (C) Murine platelets were isolated by gel filtration from WT and MyD88−/− mice and were stimulated with CAP-PEs (CHP-PE); integrin αIIbβ3 activation was assessed by FACS analysis. (D) HEK-Blue-TLR2 cells were preincubated with MyD88 BP and CP for 12 hours and then stimulated with CAP-PEs (CEP-PE or CHP-PE) for 16 to 18 hours. (E) A total of 40 μg of total protein from TLR2-overexpressing cells and control cells (Null) were separated by SDS-PAGE and immunoblotted against MyD88 antibody. (F,H) Gel-filtered human platelets were stimulated with CAP-PEs (CEP-PE or CHP-PE), lysed, and (F-G) 300 μg of lysate protein was immunoprecipitated (IP) with (F) TLR2 antibody or (G) MyD88 antibody and immunoblotted against (F) TLR1 or (G) TLR2; (H) 50 μg of total protein was separated by SDS-PAGE and TLR2, and MyD88 and actin were detected by western blotting. ***P < .001, **P < .01, *P < .05, comparing either blocking vs control peptide or WT vs knockout mice or control vs treated.
TLR2, TLR1, and MyD88 are forming a complex in response to CAP-PEs
TLR2 associates with its coreceptor TLR1 to activate the downstream signaling pathways. Coimmunoprecipitation assay demonstrated an increased interaction between TLR1 and TLR2 in human platelets stimulated with CAP-PEs (Figure 4F). We also detected presence of MyD88 in the complex with TLR1-TLR2 (Figure 4G), an event known to be critical for inducing signaling downstream of TLR1/TLR2 complex. The equal protein level in the lysates was confirmed by western blot analysis (Figure 4H).
IRAK4 and TRAF6 are involved in CAP-PEs–induced platelet activation
Interleukin-1 receptor-associated kinase 4 (IRAK4) is essential for the TLR signaling cascade.22 It associates with MyD88, leading to its phosphorylation and activation. We observed a time-dependent increase in IRAK4 phosphorylation in platelets in response to CAP-PEs. A significant increase in phosphorylation was observed as early as 2 minutes, with a maximum reached at 5 to 10 minutes (Figure 5A), suggesting that CAP-PE–induced platelet activation via MyD88-TIRAP involves IRAK4 activation. Downstream to IRAK4, TRAF6 plays role in the TLR signaling pathway. We observed a complete inhibition of CAP-PE–induced platelet activation by the TRAF6 blocking peptide, whereas the control peptide had no effect (Figure 5B).
IRAK4, TRAF6, and SFKs are involved in signaling pathway induced by CAP-PEs. (A) Gel-filtered human platelets were stimulated with CAP-PEs (CHP-PE) for the indicated times. Phosphorylation of IRAK4 was detected by western blotting. Gel-filtered human platelets were preincubated with (B) 10 μM TRAF6 blocking peptide (TRAF6 BP) or CP, (C) pan-Src kinase family inhibitor PP2 (10 μM), followed by CAP-PEs (CEP-PE) stimulation. P-selectin expression was assessed by FACS analysis. (D-E) Murine platelets were isolated by gel filtration from (D) Lyn−/− and (E) Src−/− mice and stimulated with CAP-PEs (CHP-PE) and platelets’ integrin αIIbβ3 activation was assessed by FACS. (F) Gel-filtered human platelets were preincubated with PP2, followed by CAP-PEs (CEP-PE) stimulation for 5 minutes. (G-H) Gel-filtered murine platelets isolated from Src−/− and Lyn−/− mice were stimulated with CAP-PEs (CHP-PE) for 5 minutes. (I-K) Gel-filtered human platelets are pretreated with either TLR2 (I) or TLR1 blocking antibody (Ab) (J) or its isotype-matched control IgG; (K) either MyD88 BP or the CP and then stimulated with CAP-PEs (CEP-PE) for 5 minutes. Src phosphorylation was determined by western blot analysis using phospho-specific antibody. ***P < .001, *P < .05, comparing either blocking antibody vs IgG or blocking vs control peptide.
IRAK4, TRAF6, and SFKs are involved in signaling pathway induced by CAP-PEs. (A) Gel-filtered human platelets were stimulated with CAP-PEs (CHP-PE) for the indicated times. Phosphorylation of IRAK4 was detected by western blotting. Gel-filtered human platelets were preincubated with (B) 10 μM TRAF6 blocking peptide (TRAF6 BP) or CP, (C) pan-Src kinase family inhibitor PP2 (10 μM), followed by CAP-PEs (CEP-PE) stimulation. P-selectin expression was assessed by FACS analysis. (D-E) Murine platelets were isolated by gel filtration from (D) Lyn−/− and (E) Src−/− mice and stimulated with CAP-PEs (CHP-PE) and platelets’ integrin αIIbβ3 activation was assessed by FACS. (F) Gel-filtered human platelets were preincubated with PP2, followed by CAP-PEs (CEP-PE) stimulation for 5 minutes. (G-H) Gel-filtered murine platelets isolated from Src−/− and Lyn−/− mice were stimulated with CAP-PEs (CHP-PE) for 5 minutes. (I-K) Gel-filtered human platelets are pretreated with either TLR2 (I) or TLR1 blocking antibody (Ab) (J) or its isotype-matched control IgG; (K) either MyD88 BP or the CP and then stimulated with CAP-PEs (CEP-PE) for 5 minutes. Src phosphorylation was determined by western blot analysis using phospho-specific antibody. ***P < .001, *P < .05, comparing either blocking antibody vs IgG or blocking vs control peptide.
Src family kinases are involved in the signaling pathway induced by CAP-PEs
The connection between the signaling pathway downstream of TLR and integrin activation is not clear. It has been reported that TRAF6 can induce activation of SFKs.23,24 SFKs are nonreceptor tyrosine kinases that are known to play a role in platelet activation and integrin-dependent outside-in signaling.25,26 We found that SFK inhibitor PP2 inhibited platelet activation by CAP-PEs (Figure 5C), demonstrating that SFKs are involved in the CAP-PE–induced signaling. There are at least 6 different SFK members expressed in platelets.27 Lyn is known to play an important role in several signaling pathways in platelets.28,29 To test whether Lyn is involved in CAP-PE–induced platelets activation, we compared activation of WT platelets and platelets from Lyn−/− mice and found no activation of Lyn−/− platelets in response to CAP-PEs (Figure 5D). At the same time, responses to ADP and thrombin were not changed in Lyn−/− mice (data not shown). We also tested the involvement of c-Src by using Src−/− mice and observed a significant reduction in platelet activation by CAP-PEs (Figure 5E), demonstrating that Src is also involved in CAP-PE–induced platelet activation. The members of SFKs are phosphorylated at specific tyrosine residues to induce downstream signaling. We observed increased phosphorylation of SFKs in response to CAP-PEs, which was blocked by PP2 (Figure 5F). We observed a complete lack of CAP-PE–induced phosphorylation of SFKs in the Lyn−/− and Src−/− mice (Figure 5G-H). To further explore the signaling mechanism, we tested whether SFK activation is TLR-dependent. TLR2-TLR1–blocking antibody, and MyD88-blocking peptide suppressed SFK phosphorylation induced by CAP-PEs (Figure 5I-K), demonstrating that SFK activation is downstream of TLR1, TLR2, and MyD88.
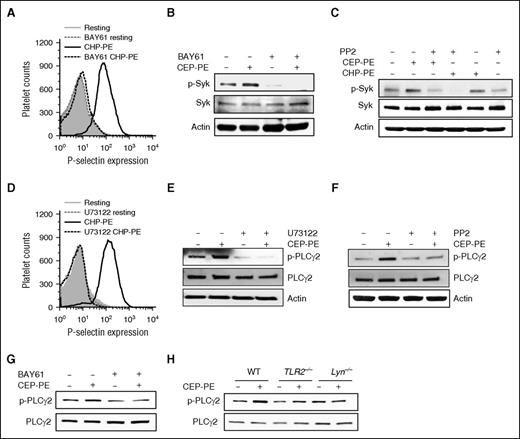
Syk and PLCγ2 are involved in the signaling pathway in platelets induced by CAP-PEs
There are reports of a collaboration between the spleen tyrosine kinase (Syk) pathway and the TLR/MyD88 pathway.30 One of the kinases that can be activated by SFKs in platelets is Syk. Syk inhibitor BAY61-3606 completely inhibited activation of human platelet by CAP-PEs (Figure 6A), demonstrating that Syk is involved in platelet activation induced by CAP-PEs. As anticipated, BAY61-3606 inhibited CAP-PEs–induced Syk autophosphorylation (Figure 6B). PP2 completely blocked the phosphorylation of Syk induced by CAP-PEs (Figure 6C), demonstrating that Syk is downstream of SFKs. PLCγ2 plays an important role in platelet activation by increasing cytoplasmic Ca2+.31 We observed complete inhibition of CAP-PE–induced human platelet activation by a general PLC-specific inhibitor U73122 (Figure 6D). Furthermore, we observed that CAP-PEs induce phosphorylation of PLCγ2, which was inhibited by U73122 (Figure 6E). PLCγ2 is a well-known target of Syk. BAY61-3606 inhibited CAP-PE–induced PLCγ2 phosphorylation (Figure 6G), demonstrating the involvement of Syk in PLCγ2 activation induced by CAP-PEs. SFK inhibitor PP2 also inhibited CAP-PE–induced PLCγ2 phosphorylation (Figure 6F). We also observed a complete abolition of CAP-PE–induced PLCγ2 phosphorylation in TLR2−/− and Lyn−/− mice (Figure 6H). Taken together, these experiments demonstrate that CAP-PEs activate platelets via TLR2-TLR1 and MyD88 dependent pathway by activating SFKs, Syk, and PLCγ2.
Syk and PLCγ2 are involved in the signaling pathway in platelets induced by CAP-PEs. (A-G) Gel-filtered human platelets were (A-B,G) preincubated with 3 μM Syk inhibitor BAY61-3606 (BAY61) or (C,F) Src kinase family inhibitor PP2 (10 μM) or (D-E) PLC inhibitor U73122 (10 μM), followed by CAP-PEs (CHP-PE or CEP-PE) stimulation. P-selectin expression was assessed by FACS analysis (A,D), Syk phosphorylation (B-C), and PLCγ2 phosphorylation (E-G) were determined by western blot analysis using phospho-specific antibodies of the respective proteins. (H) Murine platelets isolated by gel filtration from WT; TLR2−/− and Lyn−/− mice were stimulated with CAP-PEs (CEP-PE) for 5 minutes. PLCγ2 phosphorylation was determined by western blot analysis using phospho-specific antibody.
Syk and PLCγ2 are involved in the signaling pathway in platelets induced by CAP-PEs. (A-G) Gel-filtered human platelets were (A-B,G) preincubated with 3 μM Syk inhibitor BAY61-3606 (BAY61) or (C,F) Src kinase family inhibitor PP2 (10 μM) or (D-E) PLC inhibitor U73122 (10 μM), followed by CAP-PEs (CHP-PE or CEP-PE) stimulation. P-selectin expression was assessed by FACS analysis (A,D), Syk phosphorylation (B-C), and PLCγ2 phosphorylation (E-G) were determined by western blot analysis using phospho-specific antibodies of the respective proteins. (H) Murine platelets isolated by gel filtration from WT; TLR2−/− and Lyn−/− mice were stimulated with CAP-PEs (CEP-PE) for 5 minutes. PLCγ2 phosphorylation was determined by western blot analysis using phospho-specific antibody.
Discussion
The presence of lipid-derived protein derivatives in vivo is observed in many pathological states. Although the biological activities and role of such derivatives in vivo have been widely studied for the past 3 decades,7,8 studies of similar derivatives of phosphatidylethanolamine are very limited to date.6,11 Evidence supports the notion that a variety of PE adducts and derivatives may form during conditions of oxidative stress in vivo.9,11,32 Moreover, recent in vitro studies have shown that PE is the preferred target for modification by some lipid aldehydes.10 However, the biological activities of such adducts and/or derivatives have just recently attracted attention and cellular receptors recognizing them are not known. In this study, we demonstrated that novel end-products of lipid peroxidation, CAP-PEs, activate platelets by inducing formation of TLR2/TLR1 complex and downstream innate immunity signaling cascade, establishing a cross-talk with integrin activation signaling pathways involving TRAF6, SFKs, Syk, and PLCγ2 (Figure 7). We further demonstrated that CAP-PEs are present in the circulation in hyperlipidemic ApoE−/− mice and that platelet TLR2 contributes to prothrombotic phenotype of hyperlipidemic ApoE−/− mice.
Proposed model showing the signaling pathways of platelet integrin αIIbβ3 activation by the novel ligand CAP-PEs.
Proposed model showing the signaling pathways of platelet integrin αIIbβ3 activation by the novel ligand CAP-PEs.
Phosphatidylethanolamine, the second-most abundant phospholipid in mammalian cell membranes, is located in the immediate vicinity to the sites of lipid peroxidation (ie, in cell membranes or in lipoproteins), making it logical to assume that PE should be at least as prone to modification by reactive lipid aldehydes as are proteins.6,9,10 Hydroxy-ω-oxoalkenoates are a relatively recently discovered group of reactive aldehydes present in oxidatively truncated phospholipids.33,34 Conformational changes induced by oxidation expose sn-2 ester bond of oxidized phospholipids and make it preferential target for PLA2.35,36 Hydrolysis by PLA2 and reaction of the resulting unesterified hydroxy-ω-oxoalkenoic acids with ε-amino groups of protein lysyl residues gives rise to CAP proteins.33 The presence of CAP proteins is shown in vivo in tissues, in circulation, and is associated with inflammation or oxidative stress.7,8 Hyperlipidemia in ApoE−/− mice fed a Western diet is associated with accumulation of oxidized phospholipids. We have previously identified a novel structurally conserved family of oxidized choline glycerophospholipids (oxPCCD36) that serve as high-affinity ligands for scavenger receptor CD36 and accumulate in the plasma of hyperlipidemic mice. oxPCCD36 contribute significantly to platelet hyperreactivity and accelerated thrombosis in hyperlipidemic mice by engaging platelet CD36.16 CD36 deficiency in mice confers significant, but incomplete, protection from hyperreactivity associated with hyperlipidemia.16 Our current findings demonstrate that the release of hydroxy-ω-oxoalkenoic acids from oxidized (phospho)lipids also generates ligand(s) that promote platelet activation, but via a different receptor and a different signaling pathway. CAP-PEs, derivatives of hydroxy-ω-oxoalkenoates are at very low levels in the circulation of relatively normolipidemic chow diet fed ApoE−/− mice. However, we observed surprisingly high levels of carboxyalkylpyrrole immunoreactivity in PE fraction of hyperlipidemic plasma, well within the limits capable to promote increased platelet responses to physiological agonists such as ADP in circulation. Hyperlipidemic ApoE−/−/CD36−/−/TLR2−/− mice have thrombosis times that are prolonged as compared with ApoE−/−/CD36−/− mice (Figure 3F) and similar to thrombosis times of WT mice (25.6 ± 1.7 vs 26 ± 1.9). This observation indicates that both types of ligands contribute to accelerated thrombosis in hyperlipidemic mice and these 2 receptors on platelets are mostly responsible for the platelet hyperreactivity in hyperlipidemia.
Oxidized glycerophospholipids, including PE, that incorporate truncated and polar residue in sn-2 position are recognized by class B scavenger receptors CD36 and SR-BI.17 In contrast, our experiments using competitive binding assay, blocking antibody, and TLR2-deficient mice demonstrate that PE with a modified head group represent a ligand for another family of pattern recognition receptors, TLRs. TLRs play a key role in innate immunity responses in response to pathogen-associated molecular patterns. More recent studies also implicate TLRs in recognition of the endogenous ligands, particularly so-called “altered-self” ligands.12,13 We have demonstrated that CAP proteins induce angiogenesis by activating TLR2 in endothelial cells7 and can engage TLR9 on platelets.8 In this study, we have shown that CAP-PEs are directly recognized by TLR2, but activation requires TLR1 and a formation of the TLR2/TLR1 complex. It has been shown that TLR2/TLR1 agonist Pam3CSK4 can activate platelets.37,38 We confirmed this observation and showed that platelet activation by Pam3CSK4 is completely abolished in TLR2−/− mice, whereas responses to convulxin and thrombin were similar to WT mice (supplemental Figure 7A-C). Interestingly, the activation of platelets by Pam3CSK4 depends on PI3K-Akt pathway,38 whereas we observed no significant effect of PI3K inhibitor LY294002 and Akt inhibitor MK2206 on CAP-PE–induced platelet activation (supplemental Figure 7D). These data suggest that platelet TLR2 can modulate thrombosis in the presence of pathogen derived as well as endogenous ligands, but that signaling pathways differ.
In delineating the signaling pathway leading to platelet integrin activation by CAP-PEs, we demonstrated that many elements of the innate immune signaling pathway are active in platelets. TIRAP mediates bridging of MyD88, a universal adaptor for TLR signaling, to the TLR complex in immune cells. We demonstrated that MyD88, associates with the TLR1/TLR2 complex and showed that blocking peptides to either TIRAP or MyD88 inhibited CAP-PE–induced platelet activation, indicating both are involved in downstream signaling events. MyD88 is shown to recruit Ser/Thr kinase IRAK4 to the complex, leading to phosphorylation of IRAK4.22 We have demonstrated phosphorylation of IRAK4 in response to CAP-PEs. Downstream to IRAKs, TRAF6 gets activated leading to activation of a downstream signaling cascade.22 Using inhibitory peptides, we have demonstrated that TRAF6 is the intermediate molecule in the signal transduction from TLR2-TLR1 in platelets.
TRAF6 is a convergence point for many downstream and upstream signals and bridges innate and adaptive immunity.24 There are several reports of interaction between TRAF6 and SFKs leading to Src activation and activation of downstream signaling.39-41 TRAF6 can colocalize with Src in membrane rafts24 and can directly interact with SFKs (c-Src and Fyn).39 It contains a proline rich, putative SH3-binding motif, required for association with c-Src.40,41 We demonstrated that SFKs/Syk/PLCγ2 pathway is activated in platelets by CAP-PEs via TRAF6-dependent pathway leading to integrin αIIbβ3 activation. Activation of SFKs downstream of GP-VI and GPIb-IX-V followed by Syk and PLCγ2 activation, is well-known pathway leading to platelet integrin αIIbβ3 activation.31,42,43 Thus, TRAF6/SFKs signaling seems to be a good candidate for connecting innate immunity signaling from TLR to integrins. SFKs perform unique as well as redundant functions. Our finding that knockout of either c-Src or Lyn results in more than 50% inhibition of platelet activation is surprising, but not unique. Complete protection of endothelial cells activation mediated by TLR4 by knockdown of either c-Src or Fyn and 70% protection by knockdown of Yes has been reported.44 It has been suggested that such effects are explained by the unique function of each kinase plays in the process.44
In summary, our studies on the novel ligands generated in conditions of oxidative stress and induction of native immunity signaling cascade in platelets may provide important insights into the mechanisms underlying prothrombotic states associated with dyslipidemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank Lifang Zhang and Miroslava Tischenko for technical assistance, Junhong Guo for her help in writing the “Methods” section, and Emelye Crehore and Nancy Fiordalisi for proofreading of the manuscript.
This work was supported in part by the National Institutes of Health, National Heart, Lung, and Blood Institute (grants HL077213, HL071625, HL053315, and P01HL073311), National Eye Institute (grant EY016813), and National Institute of General Medicine Sciences (grant GM021249); and an American Heart Association postdoctoral fellowship grant (14POST20230027).
Authorship
Contribution: S.B. designed the research, performed the experiments, analyzed the data, and wrote the manuscript; S.P., A.Z., H.W., and V.P.Y. performed experiments and analyzed the data; L.X. synthesized CAP-PEs; R.G.S. proposed investigating the biological effects of CAP-PEs on platelets and thrombosis, designed and oversaw the synthesis and quality of the preparation of CAP-PEs, and edited the manuscript; and T.V.B. and E.A.P. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eugene A. Podrez, Cleveland Clinic, Lerner Research Institute, Department of Molecular Cardiology, NB 5-87, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: podreze@ccf.org.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal