Key Points
Donor age and donor-recipient HLA match predict survival after hematopoietic cell transplantation.
Abstract
There are >24 million registered adult donors, and the numbers of unrelated donor transplantations are increasing. The optimal strategy for prioritizing among comparably HLA-matched potential donors has not been established. Therefore, the objective of the current analyses was to study the association between donor characteristics (age, sex, parity, cytomegalovirus serostatus, HLA match, and blood group ABO match) and survival after transplantation for hematologic malignancy. The association of donor characteristics with transplantation outcomes was examined using either logistic or Cox regression models, adjusting for patient disease and transplantation characteristics associated with outcomes in 2 independent datasets: 1988 to 2006 (N = 6349; training cohort) and 2007 to 2011 (N = 4690; validation cohort). All donor-recipient pairs had allele-level HLA typing at HLA-A, -B, -C, and -DRB1, which is the current standard for selecting donors. Adjusting for patient disease and transplantation characteristics, survival was better after transplantation of grafts from young donors (aged 18-32 years) who were HLA matched to recipients (P < .001). These findings were validated for transplantations that occurred between 2007 and 2011. For every 10-year increment in donor age, there is a 5.5% increase in the hazard ratio for overall mortality. Increasing HLA disparity was also associated with worsening survival. Donor age and donor-recipient HLA match are important when selecting adult unrelated donors. Other donor characteristics such as sex, parity, and cytomegalovirus serostatus were not associated with survival. The effect of ABO matching on survival is modest and must be studied further before definitive recommendations can be offered.
Introduction
More than 24 million adult volunteers have been recruited into donor registries worldwide for patients in need of a hematopoietic stem cell transplant but lack an HLA matched sibling.1 Although there is agreement that donor-recipient HLA match is an important criterion and that HLA matching should consider allele-level HLA match at HLA-A, -B, -C, and -DRB1 loci,2 strategies vary when prioritizing among comparably HLA-matched potential donors. An earlier study of >6000 bone marrow transplantations facilitated by the National Marrow Donor Program suggested that donor age is an important factor with younger donors associated, with better overall survival and lower rates of acute and chronic graft-versus-host disease (GVHD).3 The criteria for donor-recipient HLA match has changed considerably since the publication of that report, which used low-resolution (antigen-level) data at HLA-A and -B and allele level at -DRB1. Subsequently, 4 reports have confirmed the importance of matching donor-recipient pairs at the HLA-C locus and allele-level HLA matching.4-7 Reports from the European Group for Blood and Marrow Transplantation (EBMT) have identified donor-recipient sex match as a predictor for chronic GVHD and survival after transplantation.8-10 The European reports did not examine for the effect of donor age or donor-recipient HLA match on transplantation outcomes, and those characteristics may be relevant in the setting of adult unrelated donor transplantation. Further, the EBMT reports an adverse effect on survival for transplantation of grafts from unrelated donors who are cytomegalovirus seropositive to recipients who are cytomegalovirus seronegative.11
Recruitment of volunteer adult donors is expensive, with budgets of >30 million dollars annually. The costs of recruitment are defrayed through revenue generated when transplantation is facilitated. We hypothesize identifying donor characteristics associated with outcomes after unrelated donor transplantation would not only optimize survival after transplantation but could potentially focus recruitment expenses more efficiently. Therefore, the current analysis was undertaken to assess the impact of donor characteristics (age, sex, parity, cytomegalovirus serostatus, and donor-recipient ABO blood group match) on survival, hematopoietic recovery, and GVHD in donors and recipients with allele-level HLA typing at HLA-A, -B, -C, and -DRB1.
Patients and methods
Data collection
The Center for International Blood and Marrow Transplant Research is a voluntary network of >450 transplant centers worldwide that reports data on consecutive transplantations. Patient, disease, and transplantation characteristics and outcome data are reported on standardized forms submitted at the time of transplantation (baseline) and at 100 days, 6 months, and annually thereafter. All patients or their guardians provided written informed consent. The Institutional Review Boards of the National Marrow Donor Program (NMDP) and the Medical College of Wisconsin approved this study.
Inclusion criteria
The analyses were limited to transplantations for hematologic malignancy, the most common indication for allogeneic transplantation. Donor-recipient pairs had to have had allele-level HLA typing at HLA-A, -B, -C, and -DRB1. Donor-recipient HLA typing was performed retrospectively by the National Marrow Donor Program at a central laboratory as previously described.12 Patients had acute myelogenous leukemia (AML), acute lymphocytic leukemia (ALL), chronic myelogenous leukemia (CML), or myelodysplastic syndrome (MDS). Recipients of prior autologous or allogeneic transplantation or ex vivo T cell-depleted or CD34-selected grafts were excluded.
End points
Overall survival was the primary end point. Death from any cause was considered an event. Neutrophil recovery was defined as achieving an absolute neutrophil count of ≥0.5 × 109/L for 3 consecutive measurements. Grade 2 to 4 acute and chronic GVHD was graded based on reports from each transplant center and using standard criteria.13,14 Nonrelapse mortality was defined as death is remission. Relapse was defined as morphologic, cytogenetic, or molecular recurrence of disease.
Statistical analysis
Overall survival was calculated using the Kaplan-Meier estimator.15 The effects of donor and patient ages on overall survival were first examined using residual plots.16 The plots showed 2 age cutoffs for donor age (32 and 50 years) and patient age (18 and 45 years), at which there were differences in overall survival. The donor and patient age groups in the multivariate models were created based on these observed differences. Further, donor age was tested as a continuous variable. Two independent data sets were available: a training cohort for transplant period 1988 to 2006 and a validation cohort for the period 2007 to 2011.
Multivariate models for the outcomes of interest were built using Cox regression models.17 Variables that attained P = .01 or less were considered significant. Donor-related variables tested in multivariate models include donor age (18-32 vs 33-50 vs >50 years), donor parity (male vs nulliparous female vs parous female), donor-recipient sex match (female donor/male recipient vs female donor/female recipient vs male donor/male recipient vs male donor/female recipient), donor-recipient race match (donor/recipient same race vs donor/recipient different race vs unclassified), donor-recipient cytomegalovirus serostatus (donor negative/recipient negative vs donor positive/recipient negative vs donor negative/recipient positive vs donor positive/recipient positive), ABO match (match vs minor mismatch vs major mismatch), and donor-recipient HLA match (8/8 vs 7/8 vs 6/8 vs 5/8 or lower). For ABO, minor mismatch was defined as a blood group O donor into an A, B, or AB recipient or a non-AB donor into an AB recipient; all others were classified as major mismatch. Recipient HLA genotypes were assigned to quartiles according to their frequency (using HLA genotypes of 4 million donors from the NMDP as reference). The variable for genotype frequency was tested in the multivariate model for overall mortality as a discrete covariate.
Patient and disease-related variables tested include age (<18 vs 18-45 vs >45 years), performance score (90-100 vs <90), disease (AML vs ALL vs CML vs MDS), and disease status (early [first complete remission or chronic phase] vs intermediate [second complete remission or chronic phase or accelerated phase vs advanced [not in remission or blast phase]). Transplantation variables included conditioning regimen intensity (myeloablative vs reduced intensity), GVHD prophylaxis (cyclosporine-containing without in vivo T-cell depletion with antithymocyte globulin or alemtuzumab vs cyclosporine-containing with in vivo T-cell depletion vs tacrolimus-containing without in vivo T-cell depletion vs tacrolimus-containing with in vivo T-cell depletion), graft source (bone marrow vs peripheral blood), cell dose (total nucleated cells [TNC] <3 × 108/kg vs TNC ≥3 × 108/kg for bone marrow vs CD34 <4.5 × 106/kg vs CD34 ≥4.5 × 106/kg peripheral blood),18,19 and year of transplant (1988-2002 vs 2003-2006). For bone marrow, total nucleated cell dose <3 × 108/kg and for peripheral blood, CD34 <4.5 × 106/kg was considered low dose. None of the variables tested violated the proportionality assumption, and there were no first-order interactions.
The training cohort included unrelated donor transplantations during the period 1988 to 2006. Applying the same selection criteria a validation cohort was generated for the period 2007 to 2011. Cox regression models were built to confirm donor characteristics associated with survival, the primary end point. Donor age was treated as a continuous variable because the effect of donor age on mortality was linear. All analyses were performed with SAS version 9.3 (Cary, NC).
Results
Donor, patient, and transplantation characteristics
Donors, patients, their disease, and transplantation characteristics for transplantations from 1988 to 2006 are shown in Table 1. The median age of donors was 35 years (range, 18-61 years), with 36%, 57%, and 7% of donors aged 18 to 32, 33 to 50, and >50 years, respectively. Male donors accounted for 62% of transplantations, and among female donors, 39% were nulliparous. The majority of donors and recipients were white. Fifty-nine percent of donor-recipient pairs were 8/8 HLA matched and 43% were ABO blood group matched. Older donors were more likely to be mismatched to their recipients (P < .001), be cytomegalovirus seropositive (P < .001), and donate grafts with lower cell dose (P < .001) compared with younger donors. Patients received myeloablative or reduced intensity conditioning regimens and a calcineurin inhibitor containing GVHD prophylaxis, and there were no differences between the donor groups. Twenty percent of patients received in vivo T-cell depletion with antithymocyte globulin or alemtuzumab. There were no significant differences in patient age, performance score, disease, disease status, transplant conditioning regimen, and GVHD between the 3 donor age groups.
Characteristics of patients, their donors, and transplantation, 1988 to 2006
| . | Donor age, years . | ||
|---|---|---|---|
| 18 to 32 . | 33 to 50 . | >50 . | |
| Number | 2260 | 3651 | 438 |
| Donor characteristics | |||
| Donor-recipient HLA-match | |||
| 8/8 matched | 1443 (64%) | 2066 (57%) | 224 (51%) |
| 7/8 matched | 487 (22%) | 960 (26%) | 137 (31%) |
| 6/8 matched | 216 (10%) | 421 (12%) | 50 (11%) |
| 5/8 matched or lower | 114 (5%) | 204 (6%) | 27 (6%) |
| Donor-recipient ABO match | |||
| Matched | 946 (42%) | 1529 (42%) | 180 (41%) |
| Minor mismatch | 591 (26%) | 885 (24%) | 115 (26%) |
| Major mismatch | 697 (31%) | 1157 (32%) | 137 (31%) |
| Not reported | 26 (1%) | 80 (2%) | 6 (1%) |
| Donor sex and parity | |||
| Male | 1338 (59%) | 2349 (64%) | 256 (58%) |
| Female, nulliparous | 639 (28%) | 297 (8%) | 24 (5%) |
| Female, parity, 1 or more | 256 (11%) | 957 (26%) | 155 (35%) |
| Not reported | 27 (1%) | 48 (1%) | 3 (1%) |
| Donor-recipient cytomegalovirus serostatus | |||
| Donor and recipient negative | 771 (34%) | 1080 (30%) | 114 (26%) |
| Donor negative, recipient positive | 788 (35%) | 1187 (33%) | 101 (23%) |
| Donor and recipient positive | 412 (18%) | 747 (20%) | 128 (29%) |
| Donor positive, recipient positive | 246 (11%) | 550 (15%) | 82 (19%) |
| Not reported | 43 (2%) | 87 (2%) | 13 (3%) |
| Patient characteristics | |||
| Patient age, years | |||
| <18 | 410 (18%) | 610(17%) | 55 (13%) |
| 18-45 | 1086 (48%) | 1807 (49%) | 240 (55%) |
| >45 | 764 (34%) | 1234 (34%) | 143 (33%) |
| Performance score | |||
| <90 | 598 (26%) | 967 (26%) | 117 (27%) |
| 90 or 100 | 1485 (66%) | 2426 (66%) | 290 (66%) |
| Not reported | 177 (8%) | 258 (7%) | 31 (7%) |
| Disease | |||
| Acute myeloid leukemia | 966 (43%) | 1407 (39%) | 160 (37%) |
| Acute lymphoblastic leukemia | 543 (24%) | 847 (23%) | 103 (24%) |
| Chronic myeloid leukemia | 531 (23%) | 1074 (29%) | 133 (30%) |
| Myelodysplastic syndrome | 220 (10%) | 323 (9%) | 42 (10%) |
| Donor status at transplantation | |||
| 1st complete remission/chronic phase | 913 (40%) | 1527 (42%) | 183 (42%) |
| 2nd complete remission/chronic/accelerated phase | 665 (29%) | 1088 (30%) | 123 (28%) |
| Relapse/blast phase/refractory anemia excessblasts | 682 (30%) | 1036 (28%) | 132 (30%) |
| Transplant characteristics | |||
| Conditioning regimen | |||
| Myeloablative | |||
| Total body irradiation containing | 1325 (58%) | 2270 (63%) | 273 (63%) |
| Non–irradiation containing | 631 (28%) | 940 (26%) | 116 (26%) |
| Reduced intensity | |||
| Low-dose total body irradiation containing | 86 (4%) | 115 (2%) | 12 (3%) |
| Non–irradiation containing | 218 (10%) | 326 (9%) | 37 (8%) |
| GVHD prophylaxis | |||
| Tacrolimus-containing | 1025 (46%) | 1552 (43%) | 194 (45%) |
| Cyclosporine-containing | 1235 (54%) | 2099 (57%) | 244 (55%) |
| In vivo T-cell depletion | |||
| Yes | 484 (22%) | 721 (20%) | 80 (18%) |
| None | 1776 (79%) | 2930 (80%) | 358 (82%) |
| Graft type | |||
| Bone marrow | 1351 (60%) | 2348 (64%) | 267 (61%) |
| Peripheral blood | 909 (40%) | 1303 (36%) | 171 (39%) |
| Cell dose | |||
| Bone marrow, total nucleated cells <3 × 108/kg | 686 (30%) | 1276 (35%) | 161 (37%) |
| Bone marrow, total nucleated cells ≥3 × 108/kg | 661 (29%) | 1054 (29%) | 104 (24%) |
| Peripheral blood, CD34 <4.5 × 106/kg | 286 (13%) | 451 (12%) | 66 (15%) |
| Peripheral blood, CD34 ≥4.5 × 106/kg | 603 (27%) | 824 (23%) | 96 (22%) |
| Not reported | 24 (1%) | 46 (1%) | 11 (3%) |
| Transplant period | |||
| 1987-2002 | 1082 (48%) | 1980 (54%) | 233 (53%) |
| 2003-2006 | 1178 (52%) | 1671 (46%) | 205 (47%) |
| Follow-up, median (range), months | 113 (6-290) | 119 (5-312) | 121 (8-290) |
| . | Donor age, years . | ||
|---|---|---|---|
| 18 to 32 . | 33 to 50 . | >50 . | |
| Number | 2260 | 3651 | 438 |
| Donor characteristics | |||
| Donor-recipient HLA-match | |||
| 8/8 matched | 1443 (64%) | 2066 (57%) | 224 (51%) |
| 7/8 matched | 487 (22%) | 960 (26%) | 137 (31%) |
| 6/8 matched | 216 (10%) | 421 (12%) | 50 (11%) |
| 5/8 matched or lower | 114 (5%) | 204 (6%) | 27 (6%) |
| Donor-recipient ABO match | |||
| Matched | 946 (42%) | 1529 (42%) | 180 (41%) |
| Minor mismatch | 591 (26%) | 885 (24%) | 115 (26%) |
| Major mismatch | 697 (31%) | 1157 (32%) | 137 (31%) |
| Not reported | 26 (1%) | 80 (2%) | 6 (1%) |
| Donor sex and parity | |||
| Male | 1338 (59%) | 2349 (64%) | 256 (58%) |
| Female, nulliparous | 639 (28%) | 297 (8%) | 24 (5%) |
| Female, parity, 1 or more | 256 (11%) | 957 (26%) | 155 (35%) |
| Not reported | 27 (1%) | 48 (1%) | 3 (1%) |
| Donor-recipient cytomegalovirus serostatus | |||
| Donor and recipient negative | 771 (34%) | 1080 (30%) | 114 (26%) |
| Donor negative, recipient positive | 788 (35%) | 1187 (33%) | 101 (23%) |
| Donor and recipient positive | 412 (18%) | 747 (20%) | 128 (29%) |
| Donor positive, recipient positive | 246 (11%) | 550 (15%) | 82 (19%) |
| Not reported | 43 (2%) | 87 (2%) | 13 (3%) |
| Patient characteristics | |||
| Patient age, years | |||
| <18 | 410 (18%) | 610(17%) | 55 (13%) |
| 18-45 | 1086 (48%) | 1807 (49%) | 240 (55%) |
| >45 | 764 (34%) | 1234 (34%) | 143 (33%) |
| Performance score | |||
| <90 | 598 (26%) | 967 (26%) | 117 (27%) |
| 90 or 100 | 1485 (66%) | 2426 (66%) | 290 (66%) |
| Not reported | 177 (8%) | 258 (7%) | 31 (7%) |
| Disease | |||
| Acute myeloid leukemia | 966 (43%) | 1407 (39%) | 160 (37%) |
| Acute lymphoblastic leukemia | 543 (24%) | 847 (23%) | 103 (24%) |
| Chronic myeloid leukemia | 531 (23%) | 1074 (29%) | 133 (30%) |
| Myelodysplastic syndrome | 220 (10%) | 323 (9%) | 42 (10%) |
| Donor status at transplantation | |||
| 1st complete remission/chronic phase | 913 (40%) | 1527 (42%) | 183 (42%) |
| 2nd complete remission/chronic/accelerated phase | 665 (29%) | 1088 (30%) | 123 (28%) |
| Relapse/blast phase/refractory anemia excessblasts | 682 (30%) | 1036 (28%) | 132 (30%) |
| Transplant characteristics | |||
| Conditioning regimen | |||
| Myeloablative | |||
| Total body irradiation containing | 1325 (58%) | 2270 (63%) | 273 (63%) |
| Non–irradiation containing | 631 (28%) | 940 (26%) | 116 (26%) |
| Reduced intensity | |||
| Low-dose total body irradiation containing | 86 (4%) | 115 (2%) | 12 (3%) |
| Non–irradiation containing | 218 (10%) | 326 (9%) | 37 (8%) |
| GVHD prophylaxis | |||
| Tacrolimus-containing | 1025 (46%) | 1552 (43%) | 194 (45%) |
| Cyclosporine-containing | 1235 (54%) | 2099 (57%) | 244 (55%) |
| In vivo T-cell depletion | |||
| Yes | 484 (22%) | 721 (20%) | 80 (18%) |
| None | 1776 (79%) | 2930 (80%) | 358 (82%) |
| Graft type | |||
| Bone marrow | 1351 (60%) | 2348 (64%) | 267 (61%) |
| Peripheral blood | 909 (40%) | 1303 (36%) | 171 (39%) |
| Cell dose | |||
| Bone marrow, total nucleated cells <3 × 108/kg | 686 (30%) | 1276 (35%) | 161 (37%) |
| Bone marrow, total nucleated cells ≥3 × 108/kg | 661 (29%) | 1054 (29%) | 104 (24%) |
| Peripheral blood, CD34 <4.5 × 106/kg | 286 (13%) | 451 (12%) | 66 (15%) |
| Peripheral blood, CD34 ≥4.5 × 106/kg | 603 (27%) | 824 (23%) | 96 (22%) |
| Not reported | 24 (1%) | 46 (1%) | 11 (3%) |
| Transplant period | |||
| 1987-2002 | 1082 (48%) | 1980 (54%) | 233 (53%) |
| 2003-2006 | 1178 (52%) | 1671 (46%) | 205 (47%) |
| Follow-up, median (range), months | 113 (6-290) | 119 (5-312) | 121 (8-290) |
Donors, patients, their disease, and transplantation characteristics for transplantations from 2007 to 2011 (validation cohort; N = 4690) are shown in Table 2. In the more recent period, 55% of donors were aged 18 to 32 years, 38% were aged 33 to 50 years, and only 6% were >50 years. Donor-recipient pairs were 8/8 or 7/8 HLA matched. Reduced intensity conditioning transplantations were more common, peripheral blood was the predominant graft type, and tacrolimus-containing GVHD prophylaxis was the more predominant GVHD prophylaxis regimen. CML accounted for <10% of transplantations. Other patient, disease, donor, and transplantation characteristics were comparable to the original cohort.
Characteristics of patients, their donors, and transplantation, 2007 to 2011
| . | Donor age, years . | ||
|---|---|---|---|
| 18 to 32 . | 33 to 50 . | >50 . | |
| Number | 2614 | 1794 | 282 |
| Donor characteristics | |||
| Donor-recipient HLA-match | |||
| 8/8 matched | 2114 (81%) | 1326 (74%) | 181 (64%) |
| 7/8 matched | 500 (19%) | 468 (26%) | 101 (36%) |
| Donor-recipient ABO match | |||
| Matched | 874 (33%) | 563 (31%) | 79 (28%) |
| Minor mismatch | 453 (17%) | 374 (21%) | 53 (19%) |
| Major mismatch | 615 (24%) | 442 (25%) | 86 (30%) |
| Not reported | 672 (26%) | 415 (23%) | 64 (23%) |
| Donor sex and parity | |||
| Male | 1789 (68%) | 1218 (68%) | 153 (54%) |
| Female, nulliparous | 645 (25%) | 110 (6%) | 26 (9%) |
| Female, parity, 1 or more | 157 (6%) | 448 (25%) | 102 (36%) |
| Not reported | 23 (<1%) | 18 (1%) | 1 (<1%) |
| Donor-recipient cytomegalovirus serostatus | |||
| Donor and recipient negative | 772 (30%) | 504 (28%) | 57 (20%) |
| Donor negative, recipient positive | 992 (38%) | 580 (32%) | 94 (33%) |
| Donor and recipient positive | 229 (9%) | 229 (13%) | 38 (13%) |
| Donor positive, recipient positive | 598 (23%) | 461 (26%) | 86 (30%) |
| Not reported | 23 (< 1%) | 20 (1%) | 7 (2%) |
| Patient characteristics | |||
| Patient age, years | |||
| <18 | 304 (12%) | 198 (11%) | 22 (8%) |
| 18 to 45 | 938 (36%) | 605 (34%) | 101 (36%) |
| >45 | 1372 (52%) | 991 (55%) | 159 (56%) |
| Performance score | |||
| <90 | 822 (31%) | 589 (33%) | 86 (30%) |
| 90 or 100 | 1702 (65%) | 1124 (63%) | 190 (67%) |
| Not reported | 90 (3%) | 81 (5%) | 6 (2%) |
| Disease | |||
| Acute myeloid leukemia | 1630 (62%) | 1122 (63%) | 174 (62%) |
| Acute lymphoblastic leukemia | 593 (23%) | 406 (23%) | 66 (23%) |
| Chronic myeloid leukemia | 194 (7%) | 139 (8%) | 22 (8%) |
| Myelodysplastic syndrome | 197 (8%) | 127 (7%) | 20 (7%) |
| Donor status at transplantation | |||
| 1st complete remission/chronic phase | 1262 (48%) | 889 (50%) | 134 (48%) |
| 2nd complete remission/chronic/accelerated phase | 684 (26%) | 445 (25%) | 74 (26%) |
| Relapse/blast phase/refractory anemia excess blasts | 668 (26%) | 460 (26%) | 74 (26%) |
| Transplant characteristics | |||
| Conditioning regimen | |||
| Myeloablative | |||
| Total body irradiation containing | 882 (34%) | 583 (32%) | 87 (31%) |
| Non–irradiation containing | 953 (36%) | 647 (36%) | 109 (39%) |
| Reduced intensity | |||
| Low dose total body irradiation containing | 180 (7%) | 122 (7%) | 15 (5%) |
| Non–irradiation containing | 599 (23%) | 442 (25%) | 71 (25%) |
| GVHD prophylaxis | |||
| Tacrolimus-containing | 2065 (79%) | 1405 (78%) | 215 (76%) |
| Cyclosporine-containing | 549 (21%) | 389 (22%) | 67 (24%) |
| In vivo T-cell depletion | |||
| Yes | 935 (36%) | 680 (38%) | 119 (42%) |
| None | 1679 (64%) | 1114 (62%) | 163 (58%) |
| Graft type | |||
| Bone marrow | 625 (24%) | 458 (26%) | 70 (25%) |
| Peripheral blood | 1989 (76%) | 1336 (74%) | 212 (75%) |
| Follow up, median (range), months | 25 (3-65) | 28 (3-62) | 25 (3-61) |
| . | Donor age, years . | ||
|---|---|---|---|
| 18 to 32 . | 33 to 50 . | >50 . | |
| Number | 2614 | 1794 | 282 |
| Donor characteristics | |||
| Donor-recipient HLA-match | |||
| 8/8 matched | 2114 (81%) | 1326 (74%) | 181 (64%) |
| 7/8 matched | 500 (19%) | 468 (26%) | 101 (36%) |
| Donor-recipient ABO match | |||
| Matched | 874 (33%) | 563 (31%) | 79 (28%) |
| Minor mismatch | 453 (17%) | 374 (21%) | 53 (19%) |
| Major mismatch | 615 (24%) | 442 (25%) | 86 (30%) |
| Not reported | 672 (26%) | 415 (23%) | 64 (23%) |
| Donor sex and parity | |||
| Male | 1789 (68%) | 1218 (68%) | 153 (54%) |
| Female, nulliparous | 645 (25%) | 110 (6%) | 26 (9%) |
| Female, parity, 1 or more | 157 (6%) | 448 (25%) | 102 (36%) |
| Not reported | 23 (<1%) | 18 (1%) | 1 (<1%) |
| Donor-recipient cytomegalovirus serostatus | |||
| Donor and recipient negative | 772 (30%) | 504 (28%) | 57 (20%) |
| Donor negative, recipient positive | 992 (38%) | 580 (32%) | 94 (33%) |
| Donor and recipient positive | 229 (9%) | 229 (13%) | 38 (13%) |
| Donor positive, recipient positive | 598 (23%) | 461 (26%) | 86 (30%) |
| Not reported | 23 (< 1%) | 20 (1%) | 7 (2%) |
| Patient characteristics | |||
| Patient age, years | |||
| <18 | 304 (12%) | 198 (11%) | 22 (8%) |
| 18 to 45 | 938 (36%) | 605 (34%) | 101 (36%) |
| >45 | 1372 (52%) | 991 (55%) | 159 (56%) |
| Performance score | |||
| <90 | 822 (31%) | 589 (33%) | 86 (30%) |
| 90 or 100 | 1702 (65%) | 1124 (63%) | 190 (67%) |
| Not reported | 90 (3%) | 81 (5%) | 6 (2%) |
| Disease | |||
| Acute myeloid leukemia | 1630 (62%) | 1122 (63%) | 174 (62%) |
| Acute lymphoblastic leukemia | 593 (23%) | 406 (23%) | 66 (23%) |
| Chronic myeloid leukemia | 194 (7%) | 139 (8%) | 22 (8%) |
| Myelodysplastic syndrome | 197 (8%) | 127 (7%) | 20 (7%) |
| Donor status at transplantation | |||
| 1st complete remission/chronic phase | 1262 (48%) | 889 (50%) | 134 (48%) |
| 2nd complete remission/chronic/accelerated phase | 684 (26%) | 445 (25%) | 74 (26%) |
| Relapse/blast phase/refractory anemia excess blasts | 668 (26%) | 460 (26%) | 74 (26%) |
| Transplant characteristics | |||
| Conditioning regimen | |||
| Myeloablative | |||
| Total body irradiation containing | 882 (34%) | 583 (32%) | 87 (31%) |
| Non–irradiation containing | 953 (36%) | 647 (36%) | 109 (39%) |
| Reduced intensity | |||
| Low dose total body irradiation containing | 180 (7%) | 122 (7%) | 15 (5%) |
| Non–irradiation containing | 599 (23%) | 442 (25%) | 71 (25%) |
| GVHD prophylaxis | |||
| Tacrolimus-containing | 2065 (79%) | 1405 (78%) | 215 (76%) |
| Cyclosporine-containing | 549 (21%) | 389 (22%) | 67 (24%) |
| In vivo T-cell depletion | |||
| Yes | 935 (36%) | 680 (38%) | 119 (42%) |
| None | 1679 (64%) | 1114 (62%) | 163 (58%) |
| Graft type | |||
| Bone marrow | 625 (24%) | 458 (26%) | 70 (25%) |
| Peripheral blood | 1989 (76%) | 1336 (74%) | 212 (75%) |
| Follow up, median (range), months | 25 (3-65) | 28 (3-62) | 25 (3-61) |
Overall survival
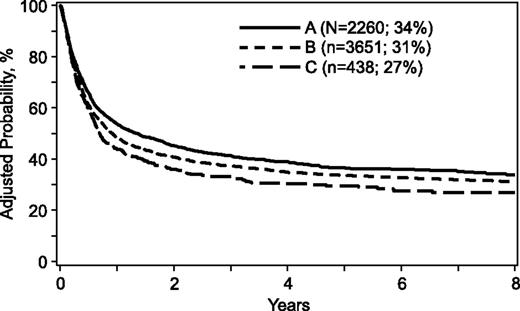
For the transplantation period 1988 to 2006, mortality risks were higher when donors were >32 years, mismatched for ABO blood group, or mismatched at 1 or more HLA loci (Table 3). A similar trend was observed when comparing donors >50 years with donors aged 33 to 50 years (hazard ration [HR], 1.15; 95% confidence interval [CI], 1.02-1.29; P = .02). Donor age tested as a continuous variable confirmed increasing age is associated with higher mortality risks (HR, 1.005; 95% CI, 1.002-1.009; P = .003). Other donor characteristics were not associated with mortality. However, several patient, disease, and transplant characteristics were associated with higher mortality (Table 4). Mortality risks were higher for patients ≥18 years of age, cytomegalovirus seropositive, performance scores <90, acute leukemia, disease status at transplantation beyond first complete remission/chronic phase, and transplant period prior to 2004. Mortality risks for cytomegalovirus seronegative recipients were not higher with transplantation of grafts from cytomegalovirus seropositive donors compared with seronegative donors (HR, 1.04; 95% CI, 0.93-1.15; P = .51). The risk-adjusted 5-year probabilities of overall survival were 36% (95% CI, 34-38), 33% (95% CI, 32-35), and 29% (95% CI, 25-33) for donors aged 18 to 32, 33 to 50, and >50 years, respectively (Figure 1). The corresponding 8-year probabilities of survival were 34% (95% CI, 31-36), 31% (95% CI, 29-32) and 27% (95% CI, 22-31).
Donor characteristics associated with mortality and GVHD for transplantation period 1988 to 2006
| Outcome . | HR (95% CI) . | P value . |
|---|---|---|
| Overall mortality* | ||
| Donor age, years | <.001 | |
| ≤32 | 1.00 | |
| 33 to 50 | 1.13 (1.05-1.20) | <.001 |
| >50 | 1.29 (1.14-1.46) | <.001 |
| Donor-recipient HLA-match | <.001 | |
| 8/8 HLA-match | 1.00 | |
| 7/8 HLA-match | 1.24 (1.15-1.34) | <.001 |
| 6/8 HLA-match | 1.62 (1.47-1.79) | <.001 |
| 5/8 or lower HLA-match | 1.89 (1.67-2.15) | <.001 |
| Blood group ABO match | .001 | |
| ABO matched | 1.00 | |
| ABO minor mismatch | 1.10 (1.01-1.18) | .002 |
| ABO major mismatch | 1.13 (1.05-1.21) | .001 |
| Nonrelapse mortality* | ||
| Donor age, years | .03 | |
| ≤32 | 1.00 | |
| 33 to 50 | 1.09 (1.01-1.19) | .03 |
| >50 | 1.20 (1.03-1.40) | .02 |
| Donor-recipient HLA-match | <.001 | |
| 8/8 HLA-match | 1.00 | |
| 7/8 HLA-match | 1.38 (1.26-1.51) | <.001 |
| 6/8 HLA-match | 1.85 (1.65-2.09) | <.001 |
| 5/8 or lower HLA-match | 2.16 (1.87-2.51) | <.001 |
| Donor sex and parity | <.001 | |
| Male | 1.00 | |
| Female, no pregnancies | 1.02 (0.91-1.14) | .75 |
| Female, 1 or more pregnancies | 1.29 (1.18-1.41) | <.001 |
| Relapse† | ||
| Donor age | .29 | |
| ≤32 y | 1.00 | |
| 33 to 50 y | 1.05 (0.95-1.16) | .35 |
| >50 y | 1.17 (0.95-1.42) | .13 |
| Donor sex and parity | .06 | |
| Male | 1.00 | |
| Female, no pregnancies | 0.96 (0.84-1.10) | .57 |
| Female, 1 or more pregnancies | 0.84 (0.74-0.95) | .007 |
| Grade 2 to 4 acute GVHD‡ | ||
| Donor age, years | .01 | |
| ≤32 | 1.00 | |
| 33 to 50 | 1.09 (1.02-1.16) | .01 |
| >50 | 1.17 (1.03-1.33) | .01 |
| Donor-recipient HLA-match | <.001 | |
| 8/8 HLA-match | 1.00 | |
| 7/8 HLA-match | 1.23 (1.14-1.32) | <.001 |
| 6/8 HLA-match | 1.26 (1.13-1.39) | <.001 |
| 5/8 or lower HLA-match | 1.46 (1.28-1.68) | <.001 |
| Chronic GVHD§ | ||
| Donor sex and parity | <.001 | |
| Male | 1.00 | |
| Female, no pregnancies | 1.01 (0.91-1.12) | .88 |
| Female, 1 or more pregnancies | 1.22 (1.11-1.34) | <.001 |
| Outcome . | HR (95% CI) . | P value . |
|---|---|---|
| Overall mortality* | ||
| Donor age, years | <.001 | |
| ≤32 | 1.00 | |
| 33 to 50 | 1.13 (1.05-1.20) | <.001 |
| >50 | 1.29 (1.14-1.46) | <.001 |
| Donor-recipient HLA-match | <.001 | |
| 8/8 HLA-match | 1.00 | |
| 7/8 HLA-match | 1.24 (1.15-1.34) | <.001 |
| 6/8 HLA-match | 1.62 (1.47-1.79) | <.001 |
| 5/8 or lower HLA-match | 1.89 (1.67-2.15) | <.001 |
| Blood group ABO match | .001 | |
| ABO matched | 1.00 | |
| ABO minor mismatch | 1.10 (1.01-1.18) | .002 |
| ABO major mismatch | 1.13 (1.05-1.21) | .001 |
| Nonrelapse mortality* | ||
| Donor age, years | .03 | |
| ≤32 | 1.00 | |
| 33 to 50 | 1.09 (1.01-1.19) | .03 |
| >50 | 1.20 (1.03-1.40) | .02 |
| Donor-recipient HLA-match | <.001 | |
| 8/8 HLA-match | 1.00 | |
| 7/8 HLA-match | 1.38 (1.26-1.51) | <.001 |
| 6/8 HLA-match | 1.85 (1.65-2.09) | <.001 |
| 5/8 or lower HLA-match | 2.16 (1.87-2.51) | <.001 |
| Donor sex and parity | <.001 | |
| Male | 1.00 | |
| Female, no pregnancies | 1.02 (0.91-1.14) | .75 |
| Female, 1 or more pregnancies | 1.29 (1.18-1.41) | <.001 |
| Relapse† | ||
| Donor age | .29 | |
| ≤32 y | 1.00 | |
| 33 to 50 y | 1.05 (0.95-1.16) | .35 |
| >50 y | 1.17 (0.95-1.42) | .13 |
| Donor sex and parity | .06 | |
| Male | 1.00 | |
| Female, no pregnancies | 0.96 (0.84-1.10) | .57 |
| Female, 1 or more pregnancies | 0.84 (0.74-0.95) | .007 |
| Grade 2 to 4 acute GVHD‡ | ||
| Donor age, years | .01 | |
| ≤32 | 1.00 | |
| 33 to 50 | 1.09 (1.02-1.16) | .01 |
| >50 | 1.17 (1.03-1.33) | .01 |
| Donor-recipient HLA-match | <.001 | |
| 8/8 HLA-match | 1.00 | |
| 7/8 HLA-match | 1.23 (1.14-1.32) | <.001 |
| 6/8 HLA-match | 1.26 (1.13-1.39) | <.001 |
| 5/8 or lower HLA-match | 1.46 (1.28-1.68) | <.001 |
| Chronic GVHD§ | ||
| Donor sex and parity | <.001 | |
| Male | 1.00 | |
| Female, no pregnancies | 1.01 (0.91-1.12) | .88 |
| Female, 1 or more pregnancies | 1.22 (1.11-1.34) | <.001 |
Model adjusted for recipient age, performance score, recipient cytomegalovirus serostatus, disease and disease status, and transplant period.
Model adjusted for performance score and disease and disease status.
Model adjusted for disease, graft type, GVHD prophylaxis, conditioning regimen intensity, and transplant period.
Model adjusted for patient age, disease, graft type, and GVHD prophylaxis.
Other factors associated with overall mortality
| Outcome . | HR (95% CI) . | P value . | |
|---|---|---|---|
| Overall mortality | |||
| Recipient age, years | <.001 | ||
| <18 | 1.00 | ||
| 18 to 45 | 1.42 (1.29-1.57) | <.001 | |
| >45 | 1.83 (1.64-2.05) | <.001 | |
| Recipient cytomegalovirus serostatus | <.001 | ||
| Seronegative | 1.00 | ||
| Seropositive | 1.24 (1.15-1.34) | <.001 | |
| Performance score | <.001 | ||
| 90 to 100 | 1.00 | ||
| <90 | 1.27 (1.19-1.37) | <.001 | |
| Disease | <.001 | ||
| Acute leukemia | 1.00 | ||
| Chronic myeloid leukemia | 0.86 (0.78-0.94) | .001 | |
| Myelodysplastic syndrome | 0.79 (0.70-0.88) | <.001 | |
| Disease status at transplantation | <.001 | ||
| 1st CR/CP/RA | 1.00 | ||
| 2nd CR/CP/AP | 1.29 (1.19-1.39) | <.001 | |
| Relapse/blast phase/RAEB | 2.10 (1.93-2.27) | <.001 | |
| Transplant period | <.001 | ||
| 2004 to 2006 | 1.00 | ||
| 1988 to 2003 | 1.22 (1.12-1.33) | <.001 |
| Outcome . | HR (95% CI) . | P value . | |
|---|---|---|---|
| Overall mortality | |||
| Recipient age, years | <.001 | ||
| <18 | 1.00 | ||
| 18 to 45 | 1.42 (1.29-1.57) | <.001 | |
| >45 | 1.83 (1.64-2.05) | <.001 | |
| Recipient cytomegalovirus serostatus | <.001 | ||
| Seronegative | 1.00 | ||
| Seropositive | 1.24 (1.15-1.34) | <.001 | |
| Performance score | <.001 | ||
| 90 to 100 | 1.00 | ||
| <90 | 1.27 (1.19-1.37) | <.001 | |
| Disease | <.001 | ||
| Acute leukemia | 1.00 | ||
| Chronic myeloid leukemia | 0.86 (0.78-0.94) | .001 | |
| Myelodysplastic syndrome | 0.79 (0.70-0.88) | <.001 | |
| Disease status at transplantation | <.001 | ||
| 1st CR/CP/RA | 1.00 | ||
| 2nd CR/CP/AP | 1.29 (1.19-1.39) | <.001 | |
| Relapse/blast phase/RAEB | 2.10 (1.93-2.27) | <.001 | |
| Transplant period | <.001 | ||
| 2004 to 2006 | 1.00 | ||
| 1988 to 2003 | 1.22 (1.12-1.33) | <.001 |
Overall survival. The risk-adjusted 5-year probabilities of overall survival were 36% (95% CI, 34-38), 33% (95% CI, 32-35), and 29% (95% CI, 25-33) for donors aged (A) 18 to 32, (B) 33 to 50, and (C) >50 years, respectively. The corresponding 8-year probabilities of survival were 34% (95% CI, 31-36), 31% (95% CI, 29-32), and 27% (95% CI, 22-31).
Overall survival. The risk-adjusted 5-year probabilities of overall survival were 36% (95% CI, 34-38), 33% (95% CI, 32-35), and 29% (95% CI, 25-33) for donors aged (A) 18 to 32, (B) 33 to 50, and (C) >50 years, respectively. The corresponding 8-year probabilities of survival were 34% (95% CI, 31-36), 31% (95% CI, 29-32), and 27% (95% CI, 22-31).
The effect of donor age on survival was independent of disease (P = .28), disease status at transplantation (P = .56), donor sex/parity (P = .81), patient age (P = .15), donor-recipient HLA match (P = .07), performance score (P = .07), graft type (P = .86), and transplant period (P = .86). The effect of donor age on mortality was also stratified by disease status, and HRs were similar across disease status; mortality risks with increasing donor age for transplantations in first complete remission/chronic phase (HR, 1.006; 95% CI, 1.001-1.012; P = .03), second complete remission/chronic/accelerated phase (HR, 1.009; 95% CI, 1.003-1.016; P = .005), and more advanced disease (HR, 1.010; 95% CI, 1.004-1.015; P = .0001). Patients who received grafts from older donors were more likely to have less common HLA genotypes. HLA genotype was not associated with overall mortality (P = .24), and the effect of donor age on mortality was independent of recipient HLA genotype (P = .79).
Validation cohort
The results of multivariate analysis for the transplantation period 2007 to 2011 are shown in Table 5. We confirmed that for every 10-year increment in donor age, there is a 5.5% increase in the HR for overall mortality after adjusting for other factors associated with overall mortality. Donor-recipient HLA match was the only donor factor associated with mortality. The estimated HR and 95% CI for the effect of ABO match on overall mortality did not meet statistical significance. Compared with ABO-matched transplants, mortality risks associated with minor and major ABO mismatched transplants were HR, 1.09; 95% CI, 0.98-1.23; P = .11 and HR, 1.01; 95% CI, 0.91-1.21; P = .83, respectively. We also considered the effect of ABO match separately for bone marrow and peripheral grafts and did not see a significant effect of ABO mismatching on overall mortality (data not shown).
Donor characteristics associated with survival for transplantation period 2007 to 2011
| Outcome . | HR (95% CI) . | P value . |
|---|---|---|
| Overall survival* | ||
| Donor age (10-year increments) | 1.055 (1.013-1.099) | .01 |
| Donor-recipient HLA-match | ||
| 8/8 HLA-match | 1.00 | |
| 7/8 HLA-match | 1.37 (1.25-1.51) | <.001 |
| Outcome . | HR (95% CI) . | P value . |
|---|---|---|
| Overall survival* | ||
| Donor age (10-year increments) | 1.055 (1.013-1.099) | .01 |
| Donor-recipient HLA-match | ||
| 8/8 HLA-match | 1.00 | |
| 7/8 HLA-match | 1.37 (1.25-1.51) | <.001 |
Adjusted for recipient age, disease, disease status, performance score, recipient, and cytomegalovirus serostatus.
Nonrelapse mortality and relapse
The effects of donor characteristics on nonrelapse mortality and relapse are shown in Table 3. Nonrelapse mortality was higher with transplantation of grafts from older donors. Donor age tested as a continuous variable confirmed increasing age is associated with higher nonrelapse mortality risks (HR, 1.005; 95% CI, 1.001-1.010; P = .02). Other donor characteristics associated with higher nonrelapse mortality included transplantation of grafts from parous female donors and donors mismatched to recipients at 1 or more HLA loci (Table 3). Similar to overall mortality, nonrelapse mortality risks were higher for patients ≥18 years of age, cytomegalovirus seropositive, poor performance score, AML, disease status at transplantation beyond first complete remission/chronic phase, and transplant period prior to 2004 (data not shown).
Donor age was not associated with relapse (Table 3). Donor age tested as a continuous variable was also not associated with relapse risks (HR, 1.004; 95% CI, 0.99-1.01; P = .20). The only donor characteristic associated with lower relapse risk was transplantation of grafts from parous females compared with male donors (Table 3). Relapse risks were higher in patients with performance score <90 compared with those with 90 to 100 (HR, 1.29; 95% CI, 1.16-1.43; P < .0001) and in second complete remission, second chronic, or accelerated phase (HR, 1.37; 95% CI, 1.21-1.56; P < .0001), or in relapse or blast phase or refractory anemia with excess blasts (HR, 3.21; 95% CI, 2.84-3.63; P < .0001) compared with transplants in first complete remission, first chronic phase, or refractory anemia. Compared with acute leukemia, relapse risks were lower for CML (HR, 0.54; 95% CI, 0.46-0.63; P < .0001), and MDS (HR, 0.45; 95% CI, 0.38-0.55; P < .0001).
Neutrophil recovery and acute and chronic GVHD
The likelihood of neutrophil recovery was lower after transplantation of allografts from female donors and from donors mismatched at 2 or more HLA loci (odds ratio [OR], 1.26; 95% CI, 1.06-1.51; P = .007). Compared with 8/8 HLA-matched transplants, likelihood of recovery was lower after 6/8 (OR, 0.72; 95% CI, 0.55-0.93; P = .01) and 5/8 (OR, 0.55; 95% CI, 0.40-0.74; P < .001) but not 7/8 HLA-matched transplants (OR, 0.85; 95% CI, 0.69-1.04; P = .11). Other donor characteristics including donor age were not associated with neutrophil recovery. However, neutrophil recovery was more likely in recipients with performance scores of 90 or 100 compared with those with scores <90 (OR, 1.67; 95% CI, 1.38-2.01; P < .001), early (OR, 1.61; 95% CI, 1.32-2.00; P < .001), and intermediate (OR, 1.64; 95% CI, 1.32-2.04; P < .001) compared with advanced disease at transplantation (ie, transplanted in relapse or blast phase). Peripheral blood grafts were associated with higher likelihoods of recovery compared with bone marrow, and among bone marrow recipients, grafts with cell dose ≥3 × 108/kg recipient body weight were associated with better recovery compared with grafts with lower cell dose (OR, 0.67; 95% CI, 0.55-0.82; P < .001).
Compared with donors 18 to 32 years of age, risks of grade 2 to 4 acute GVHD were higher with transplantation of grafts from donors ≤33 years of age (Table 3). Risks of grade 2 to 4 acute GVHD did not differ significantly with transplantation of grafts from donors >50 years compared with those aged 33 to 50 years. Donor-recipient HLA mismatching was the only other donor characteristic associated with higher risks of grade 2 to 4 acute GVHD. Other characteristics associated with higher risks for acute GVHD included myeloablative compared with reduced intensity transplant conditioning (HR 1.26, 95% CI 1.12 – 1.41, P < .001), cyclosporine-containing compared with tacrolimus-containing GVHD prophylaxis (HR, 1.28; 95% CI, 1.19-1.39; P < .001), peripheral blood compared with bone marrow (HR, 1.32; 95% CI, 1.21-1.43, P < .001), and transplantations prior to 2004 (HR, 1.15; 95% CI, 1.05-1.25; P < .001). There were no differences in acute grade 2 to 4 GVHD risks between cyclosporine-containing and tacrolimus-containing regimens when anti-thymocyte globulin was included (HR, 0.89; 95% CI, 0.76-1.04; P = .14).
The only donor characteristic associated with chronic GVHD was donor parity; risks were higher with female parous donors compared with male donors (Table 3). Chronic GVHD risks were not different after transplantation of grafts from nulliparous females compared with males. Donor age, donor-recipient HLA match, and blood group ABO compatibility was not associated with chronic GVHD. There were several patient, disease, and transplantation characteristics associated chronic GVHD risks. Compared with patients <18 years, risks were higher for those aged 18 to 45 years (HR, 1.22; 95% CI, 1.09-1.37; P < .001) and >45 years (HR, 1.24; 95% CI, 1.09-1.41; P = .001). Patients with CML were at higher risk compared with those with AML (HR, 1.33; 95% CI, 1.21-1.46; P = .001) and ALL (HR, 1.30; 95% CI, 1.18-1.43; P < .001) but not MDS (HR, 1.08; 95% CI, 0.95-1.25; P = .19). Transplantation of peripheral blood led to higher risks compared with bone marrow (HR, 1.44; 95% CI, 1.32-1.57; P < .001). Risks were also higher with cyclosporine-containing GVHD prophylaxis without anti-thymocyte globulin (HR, 1.22; 95% CI, 1.12-1.33; P < .001) and with anti-thymocyte globulin (HR, 1.23; 95% CI, 1.06-1.43; P = .004) compared with the corresponding tacrolimus-containing GVHD prophylaxis.
Discussion
With increasing numbers of transplantations being performed with grafts from adult unrelated donors, it is critical to identify donor characteristics associated with survival after transplantation after adjusting for relevant patient, disease, and transplantation characteristics. An early report from the NMDP identified older donors and donor-recipient HLA disparity to have an adverse effect on survival,3 but 4 relatively recent reports that focused on the effects of better donor-recipient HLA matching on survival, ie, matching at the allele level, did not show an effect of donor age on survival.4-7 Therefore, the current analyses were undertaken to specifically explore potential effects of donor characteristics on outcomes of unrelated donor hematopoietic cell transplantation in a cohort of 10 000 recipients with well-characterized HLA-A, -B, -C, and -DRB1 matching. The current analyses confirm younger HLA-matched donors had the best survival rates after adjusting for patient, disease, and transplant characteristics. Older donor transplantations were also associated with higher nonrelapse mortality but donor age was not associated with relapse. Donor cytomegalovirus serostatus was not associated with survival.
The importance of HLA matching is well known, and priority is given to identifying the best available HLA-matched donor. Our study population suggests donors >50 years are avoided, with most transplants using donors aged 33 to 50 years in the earlier period and, more recently, donors aged 18 to 32 years. Although we observed higher mortality associated with blood group ABO mismatched transplants prior to 2007, this was not the case in the more recent cohort. The Japan Marrow Donor Program reported 1-year survival of 63% after ABO-matched transplantation compared with 57% after minor and major ABO mismatched transplantations.20 However, hematopoietic progenitor cells do not express ABO antigens, and it is the absence of these antigens that allows for engraftment of donor cells in the marrow. Therefore, the mechanism leading to lower survival with ABO blood incompatibility is not easily explained.
As marrow cellularity deteriorates with age, grafts from older donors yield fewer cells.21 However, we failed to see an association between transplantation of grafts with relatively low cell dose and survival. Donor registries dictate a minimum accepted cell dose, and most collections comply with the minimum required standards. Others have studied the association between graft cellular composition (myeloid, lymphoid, and activated lymphoid cells) and outcomes after transplantations.22 In that report, cellular composition was not associated with neutrophil recovery, GVHD, or survival after transplantation of bone marrow. However, higher CD34 dose of peripheral blood grafts was associated with better survival but had no effect on neutrophil recovery or GVHD.18,22 Our data suggest 60% to 70% of grafts from peripheral blood donors across the 3 age groups achieved the desired CD34 dose.
The observed higher rates of grade 2 to 4 acute GVHD after transplantation of grafts from older donors may be explained by replacement of naïve T cells with memory T cells as the immune system ages in the older donors.21 Donor age was not associated with chronic GVHD and consistent with other reports. Our analyses support donor parity rather than the traditional donor-recipient sex match combination is associated with chronic GVHD, nonrelapse mortality, and relapse. However, any benefit from lower relapse risks associated with transplantation of grafts from female parous donor was negated by higher nonrelapse mortality. This differs from EBMT reports that support higher chronic GVHD and lower survival with transplantation of grafts from female donors to male recipients.8-10 The observed differences between the current analyses and the EBMT reports can be attributed to differences in study populations. The current analysis is exclusively unrelated donor transplantations with donor-recipient HLA matching at the allele level. The EBMT reports make a distinction between related and unrelated donors, but donor-recipient HLA match, an important prognostic factor for survival for unrelated donor transplantation, is not considered.
Various strategies are used to select an adult unrelated donor when multiple suitably HLA-matched donors are available. Our findings support including donor age to the selection algorithm. Optimizing donor selection by blood ABO matching must be studied further before definitive recommendations can be offered. We acknowledge the likelihood of identifying a fully HLA-matched donor for nonwhites is substantially lower than for whites, and incorporating donor age to the selection algorithm may mitigate some of the excess mortality associated with HLA-mismatched transplantations. In the recent report by Gragert et al,1 the likelihood of identifying an 8/8 or 7/8 HLA-matched donor is 97% for European whites and 76% for African Americans. Restricting the adult donor pool to those aged 18 to 32 years within the current donor registry, the likelihood of identifying an 8/8 or 7/8 HLA-matched donor shows a 3% decrement (from 97% to 94%) for European whites and a higher decrement of 18% (from 76% to 58%) for African Americans (M. Maiers and S. Spellman, personal communication, December 2014). Alternative donors such as unrelated umbilical cord blood and haploidentical related donors should ensure patients with rare HLA genotypes are not denied access to transplantation.1,23-25
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The Center for International Blood and Marrow Transplant Research is supported by Public Health Service grant U24-CA076518 from the National Cancer Institute, the National Heart, Lung and Blood Institute, and the National Institute of Allergy and Infectious Diseases; contract HHSH250201200016C with Health Resources and Services Administration; grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from Actinium Pharmaceuticals; Allos Therapeutics, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Celgene Corporation; Chimerix, Inc.; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc.; Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Kiadis Pharma; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; Sanofi US; Seattle Genetics; Sigma-τ Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; TerumoBCT; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration, or any other agency of the US Government.
Authorship
Contribution: C. Kollman, S.R.S., D.L.C., C.K.H., M.M.H., and M.E. designed the study; A.H. assembled the data; M.-J.Z. performed statistical analysis; C. Kollman drafted the manuscript; S.R.S., M.-J.Z., A.H., C.A., J.H.A., R.E.C., D.L.C., J.F.D., M.F.-V., R.J.H., M.M.H., C.K.H., C. Karanes, M.M., C.R.M., M.-A.P., M.S., A.E.W., N.Y., and M.E. critically reviewed and edited the manuscript; and all authors approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary Eapen, Center for International Blood and Marrow Transplant Research, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI 53226; e-mail: meapen@mcw.edu.