Key Points
Different point mutations in the PML moiety of PML-RARA mediate varying responses to arsenic treatment.
Increasing the concentration of arsenic trioxide or combining it with ATRA may overcome the arsenic resistance driven by the acquired point mutations.
Abstract
Resistance to arsenic and/or all-trans retinoic acid (ATRA) is a challenging problem in the clinical management of acute promyelocytic leukemia (APL). Acquired genetic mutations in the PML moiety of the PML-RARA fusion gene are found in some patients with relapsed/refractory APL. Whether all of the identified point mutations play a role and have a similar function in the mechanisms of arsenic resistance remains unknown. Here we performed in vitro functional analyses and a retrospective analysis of APL patients to investigate the effect of PML-RARA mutations in mediating resistance to arsenic trioxide. Among the 5-point mutations in the PML part of PML-RARA identified in patients with relapsed APL, we found that A216V, S214L, and A216T mutations could attenuate the negative regulation of arsenic on PML-RARA, resulting in the retention of oncoproteins. In contrast, L217F and S220G mutations functioned weakly in this context. Furthermore, we demonstrated that either increasing the concentration of arsenic trioxide or combining it with ATRA could overcome the mutation-triggered arsenic resistance in vitro. In addition to presenting more evidence to reinforce the correlation of genetic mutations in PML-RARA with arsenic efficacy, we provide novel insight into the functional difference of acquired mutations of PML-RARA both in vitro and in the clinical setting. Our findings may help predict the prognosis and select more effective strategies during APL therapy.
Introduction
Expression of the promyelocytic leukemia (PML)–retinoic acid receptor-α (RARA) fusion gene initiates acute promyelocytic leukemia (APL) and plays a crucial role in APL pathogenesis. Arsenic trioxide (As2O3) cures APL by targeting the PML-RARA oncoprotein for degradation, thereby eradicating the malignant cells. An increasing amount of evidence supports the use of As2O3 as a front-line drug in APL treatment, and the overall survival of APL patients has improved dramatically with this treatment.1-4 Unfortunately, some patients still develop arsenic resistance during therapy, and their outcomes are extremely poor.
Previous studies have confirmed that As2O3 binds directly to cysteine residues located within the RBCC motif of PML/PML-RARA, which constitutes the molecular basis of arsenic regulation of the fate of PML-RARA proteins.5 Although C212/C213 mutations in the PML B2 domain of PML-RARA can abolish the effect of arsenic on target proteins in vitro,6 A216V and L218P mutations in the PML moiety of PML-RARA were detectable in 2 APL patients with resistance to arsenic therapy.7 More recently, we identified a panel of point mutations (including the previously reported A216V and newly identified S214L, A216T, L217F, and S220G) in 9 of 13 patients with APL and resistance to arsenic.8 These findings indicated the existence of a mutational hotspot (S214-S220) within the PML-RARA oncoprotein among APL patients at relapse. Whether each of the identified point mutations functions distinctly in the mechanism underlying the resistance to arsenic therapy is of particular interest.
In the current study, we evaluated the functional differences in the identified genetic mutations within PML-RARA and attempted to overcome mutation-triggered arsenic resistance. Our findings could be translationally important, helping to improve therapeutic strategies for patients with relapsed APL.
Methods
Plasmid construction, cell culture, and transfection
The wild-type PML-RARA DNA sequence was amplified using polymerase chain reaction (PCR) from cDNA samples of patients with APL. The coding sequence of PML-RARA was then constructed into the Flag-tagged pCAG expression vector. The PML-RARA mutant fragments with A216V, S214L, A216T, L217F, or S220G substitutions were generated by PCR amplification and constructed into the same expression vectors. Hemagglutinin (HA)-tagged SUMO-2 was purchased from Addgene. HeLa cells were maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum at 37°C in 5% CO2. For protein expression experiments, HeLa cells (2.5 ×105/well) were cultured in 6-well plates and transfected with the indicated plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Transfected cells were treated with different concentrations of As2O3 (1 μM, 2.5 μM, 5uM, or 10 μM) with or without 1 μM of ATRA (Sigma) at 28 hours after transfection for different time courses as indicated in the text.
Immunoblot analyses
The whole-cell protein lysates for immunoblotting were harvested using 1% NP-40 lysis buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1% NP-40, and a complete protease and phosphatase inhibitor cocktail). After centrifugation, the supernatants were transferred into new tubes as the soluble fractions. To obtain the insoluble fractions of PML-RARA proteins, 150 μL of phosphate-buffered saline plus 4× sodium dodecyl sulfate (SDS) loading buffer were added to the pellets. Then the resuspended pellets were boiled for 5 minutes followed by centrifugation. Both soluble (25 μg of total lysates plus 4× SDS loading buffer) and insoluble samples (half the volume of the loading amount of the soluble fraction) were subjected to 6% SDS polyacrylamide gel electrophoresis. Immunoblotting was then carried out with the following primary antibodies: mouse anti-Flag (Cell Signaling), rabbit anti-HA (Cell Signaling), and mouse anti-α-Tubulin (Sigma).
Immunofluorescence microscopy
HeLa cells were cultured on coverslips and transfected with Flag-tagged PML-RARA and its mutants. After treatment with or without As2O3, cells were fixed with methanol for 20 minutes at –20°C. After blocking in phosphate-buffered saline with 10% goat serum for 30 minutes at room temperature, coverslips were incubated with mouse anti-Flag antibody (1:1000) at 4°C overnight. The immunofluorescent signal was then developed with Alexa Fluor 546 Donkey Anti-Mouse immunoglobulin (1:200, Life Technology) for 2 hours at room temperature. The coverslips were mounted and examined under a TCSSP5 laser-scanning confocal microscope with a 40 version lens (Leica Microsystems, Germany).
Lentivirus production and cell infection
The long isoform of wild-type PML-RARA and its mutants with A216T or S220G were subcloned from Flag-tagged pCAG constructs into the human lentiviral expression vector pLVX-MCS. After packaging, the lentiviral supernatants were collected for transduction.
Bone marrow mononuclear cells of healthy donors were isolated via standard Ficoll-Hypaque density gradient centrifugation. Then CD34+ hematopoietic stem cells (HSCs) were purified by positive selection using the Miltenyi MACS CD34+ cell isolation kit according to the manufacturer’s instructions (Miltenyi Biotec). CD34+ cells were then seeded in 6-well plates at 6 × 105 cells per well in CellGro SCGM Medium (CellGenix Inc.). U937 cells, a human leukemia cell line, were cultured in a 6-well plate with RPMI 1640 containing 10% fetal bovine serum.
Both CD34+ HSCs and U937 cells were transduced with the lentiviral expression constructs using a multiplicity of infection of 100. Infected cells were treated with different concentrations of As2O3 or combined As2O3-ATRA for different time points. Protein samples were then isolated for immunoblotting analyses as described before.
Patients
As described previously,8 5-point mutations in the PML B2 domain of PML-RARA transcripts were identified from 9 patients who were diagnosed with APL and as resistant to arsenic. All of these patients received reinduction treatment combining arsenic with ATRA at the time of disease relapse. Relapse was diagnosed according to the criteria recommended by European LeukemiaNet on 2010.9 The morphologic characteristics and expression levels of PML-RARA transcripts in the bone marrow of included patients were monitored before and after reinduction treatment with arsenic. All procedures were conducted according to the protocols approved by the Ethical Committee of Peking University Institute of Hematology.
Results
Construction and expression of wild-type and mutant PML-RARA in HeLa cells
Schematic structures of the long and short isoforms of PML-RARA are shown in Figure 1A. In the current study, using quantitative reverse transcription–PCR analysis, all 5-point mutations (A216V, S214L, A216T, L217F, and S220G) that we identified recently were found within the long isoform of PML-RARA transcripts from APL patients with arsenic resistance. In contrast, A216V and A216T mutations were found within the short isoform of PML-RARA in patients with relapsed APL.
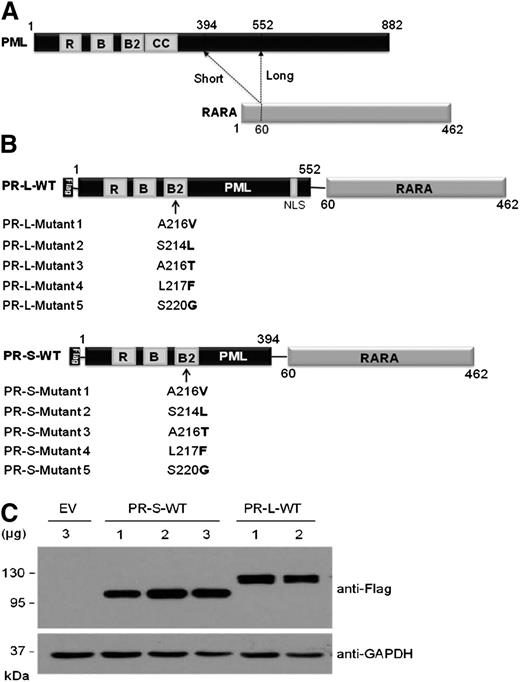
Construction and expression of the Flag-tagged PML-RARA fusion protein. (A) Schematic for 2 major isoforms of PML-RARA fusion proteins. Functional domains within the PML moiety are indicated. The dashed lines indicate the break points in the fusion proteins. RING, the RING finger; B1 and B2, B-box motifs; C-C, coiled-coil. (B) Schematic representation for the Flag-tagged long (top) and short (bottom) isoforms of the PML-RARA fusion proteins with the indicated point mutations. Bold letters indicate amino acid substitutions inside the B2 domain of the PML part. (C) Immunoblotting analysis for the expression levels of the short and long isoforms of Flag-tagged PML-RARA (PR-S-WT and PR-L-WT, respectively) in HeLa cells transfected with the indicated doses of plasmids. The levels of endogenous Tubulin expression were detected as loading controls.
Construction and expression of the Flag-tagged PML-RARA fusion protein. (A) Schematic for 2 major isoforms of PML-RARA fusion proteins. Functional domains within the PML moiety are indicated. The dashed lines indicate the break points in the fusion proteins. RING, the RING finger; B1 and B2, B-box motifs; C-C, coiled-coil. (B) Schematic representation for the Flag-tagged long (top) and short (bottom) isoforms of the PML-RARA fusion proteins with the indicated point mutations. Bold letters indicate amino acid substitutions inside the B2 domain of the PML part. (C) Immunoblotting analysis for the expression levels of the short and long isoforms of Flag-tagged PML-RARA (PR-S-WT and PR-L-WT, respectively) in HeLa cells transfected with the indicated doses of plasmids. The levels of endogenous Tubulin expression were detected as loading controls.
To explore whether the identified point mutations play a role in the mechanism underlying clinical arsenic resistance, both the long and short isoforms of wild-type or mutant PML-RARA DNA sequences were constructed into the expression vector with the Flag tag (Figure 1B). Overexpression of the long and short isoforms of wild-type PML-RARA fusion proteins (referred to as PR-L-WT and PR-S-WT, respectively) in cultured HeLa cells was confirmed by immunoblotting analysis (Figure 1C).
Varying responses to arsenic treatment in PML-RARA oncoproteins with the indicated point mutations
Next, we investigated the effect of arsenic on recombinant PML-RARA with different point mutations. The concentrations of As2O3 used in previous studies that elucidated the effect of arsenic modulation on the fate of PML-RARA varied, but all were ≥1 μM.5-7 In line with earlier findings, exposure to either 1 μM or 10 μM of As2O3 for the indicated time courses induced marked reduction of PR-L-WT in HeLa cells (Figure 2A).
Varying degrees of PML-RARA with different point mutations in response to As2O3 treatment. (A) Immunoblotting analyses for the expression of the long isoform of wild-type PML-RARA in HeLa cells treated with or without As2O3 for the indicated time course. The expression levels of endogenous Tubulin were detected as loading controls. (B) Immunoblotting analysis for the expression of Flag-tagged wild-type (PR-L-WT) and mutant PML-RARA in HeLa cells with or without As2O3 treatment (1 μM, 16 hours). (C) Immunoblotting analysis for the expression of Flag-tagged L217F and S220G mutants in HeLa cells treated with or without As2O3 (0.5 μM, 16 hours).
Varying degrees of PML-RARA with different point mutations in response to As2O3 treatment. (A) Immunoblotting analyses for the expression of the long isoform of wild-type PML-RARA in HeLa cells treated with or without As2O3 for the indicated time course. The expression levels of endogenous Tubulin were detected as loading controls. (B) Immunoblotting analysis for the expression of Flag-tagged wild-type (PR-L-WT) and mutant PML-RARA in HeLa cells with or without As2O3 treatment (1 μM, 16 hours). (C) Immunoblotting analysis for the expression of Flag-tagged L217F and S220G mutants in HeLa cells treated with or without As2O3 (0.5 μM, 16 hours).
Interestingly, our immunoblotting analyses demonstrated that expression of L217F and S220G mutants was inhibited by As2O3 (1 μM, 16 hours), which is similar to the phenotype of PR-L-WT (Figure 2B). As the As2O3 concentration was further decreased (0.5 μM, 16 hours), total protein levels of L217F and S220G mutants were still decreased markedly and accompanied by increased multimerization in their detergent-insoluble fractions (Figure 2C). In contrast, A216V, S214L, and A216T mutants were apparently arsenic-resistant and not degraded under the same experimental conditions (Figure 2B). These results indicated that As2O3 could induce a shift of PR-L mutants with L217F or S220G, but not with A216V, S214L, or A216T, from the supernatant of cell lysates to the detergent-insoluble pellet, further leading to the degradation of targeted proteins.
Different patterns of nuclear localization and SUMOylation in PML-RARA mutants upon arsenic treatment
Arsenic-triggered degradation is intimately associated with the translocation of PML/PML-RARA in the nucleus and the formation of a special nuclear organelle, called nuclear bodies.10,11 We performed immunofluorescence staining to examine the cellular localization of PR-L-WT and its mutants. As reported previously, overexpressed PR-L-WT proteins localized in the nucleus, showing a speckled pattern that shifted to a macrogranular pattern (representing the association with nuclear matrix and formation of nuclear bodies) upon exposure of As2O3 in HeLa cells (Figure 3A). In parallel with the observations from the immunoblotting assay (Figure 2B), arsenic treatment did not alter the nuclear localization of PR-L mutants with A216V, S214L, or A216T (Figure 3B-D). However, As2O3 did trigger the association of L217F and S220G mutants with the nuclear matrix (Figure 3E-F, white arrows).
Varying cellular localization of mutant PML-RARA upon As2O3 treatment compared with wild-type. Representative immunofluorescence analysis for Flag-tagged wild-type (A) and mutant (B-F) PML-RARA expressed in HeLa cells, with or without As2O3 treatment (1 μM, 16 hours). The staining of Flag is shown in red. 4,6 diamidino-2-phenylindole (DAPI) is shown in blue.
Varying cellular localization of mutant PML-RARA upon As2O3 treatment compared with wild-type. Representative immunofluorescence analysis for Flag-tagged wild-type (A) and mutant (B-F) PML-RARA expressed in HeLa cells, with or without As2O3 treatment (1 μM, 16 hours). The staining of Flag is shown in red. 4,6 diamidino-2-phenylindole (DAPI) is shown in blue.
Statistical analysis was performed for 100 Flag+ nuclei in each condition. In PR-L-WT cells, a macrogranular pattern was found in 87% of nuclei upon As2O3 treatment compared with 15% of nuclei without As2O3 (P < .001, data not shown). The representative phenotype was not observed in the A216V, S214L, or A216T mutant cells, with or without arsenic. In contrast, nuclear translocation was found, respectively, in 86% and 91% of L217F and S220G transfected cells in the presence of As2O3, compared with 5% and 9% in control cells without As2O3 (P < .001, respectively). A similar phenotype of nuclear localization was observed in the short isoform of PML-RARA with the indicated mutations (data not shown).
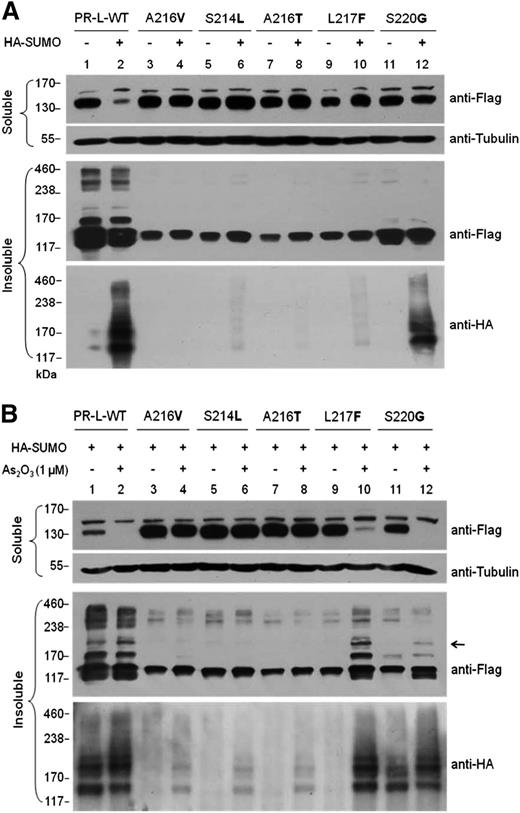
Conversely, posttranslational SUMOylation of PML/PML-RARA is reported to be critical in responsiveness to As2O3.12,13 To investigate the functional differences among PR-L-WT and its mutants, HA-tagged small ubiquitin-like protein modifier (SUMO-2) or its negative control was cotransfected with Flag-tagged PR-L plasmids into HeLa cells. In the presence of SUMO, compared with negative control, total protein levels of PR-L-WT were decreased, and SUMOylation in the insoluble fraction was induced (Figure 4A, lanes 1 and 2). Coexpression of SUMO did not trigger the protein degradation of PML-RARA with different mutations (Figure 4A, lanes 3-12). The intensity of SUMOylated bands was enhanced in the insoluble fractions of PR-L-WT with As2O3 treatment (Figure 4B, lane 2, middle and bottom rows), which accounted for the significant degradation of the total protein in its soluble fraction (Figure 4B, lane 2, top row). We did not observe the SUMOylated bands in PR-L mutants with A216V, S214L, or A216T, with or without As2O3. Accordingly, the total protein levels of these mutants were not changed (Figure 4B, lanes 3-8). In contrast, the mobility-shifted bands were clearly found in L217F and S220G mutants upon As2O3 exposure, consistent with the significant reduction in their total protein levels (Figure 4B, lanes 9-12). These results suggest that A216V, S214L, and A216T mutations could attenuate the posttranslational modification of PML-RARA upon arsenic treatment, leading to the retention of oncoproteins. In contrast, L217F and S220G mutations resulted in less resistance in this context.
Functional difference of acquired mutations in driving the SUMOylation of PML-RARA proteins in HeLa cells. Immunoblotting analyses for (A) Flag-tagged PR-L-WT and its mutants cotransfected with HA-tagged SUMO-2 or its negative control in HeLa cells; (B) Flag-tagged PR-L-WT and its mutants cotransfected with HA-tagged SUMO-2, respectively, followed by treatment with or without As2O3 (1 μM, 16 hours) in HeLa cells. Top row, protein levels in the soluble fraction isolated from whole-cell lysates were detected by anti-Flag and anti-Tubulin antibodies. Middle and bottom rows, protein levels in detergent-insoluble pellets from whole-cell lysates were detected by anti-Flag and anti-HA primary antibodies, respectively.
Functional difference of acquired mutations in driving the SUMOylation of PML-RARA proteins in HeLa cells. Immunoblotting analyses for (A) Flag-tagged PR-L-WT and its mutants cotransfected with HA-tagged SUMO-2 or its negative control in HeLa cells; (B) Flag-tagged PR-L-WT and its mutants cotransfected with HA-tagged SUMO-2, respectively, followed by treatment with or without As2O3 (1 μM, 16 hours) in HeLa cells. Top row, protein levels in the soluble fraction isolated from whole-cell lysates were detected by anti-Flag and anti-Tubulin antibodies. Middle and bottom rows, protein levels in detergent-insoluble pellets from whole-cell lysates were detected by anti-Flag and anti-HA primary antibodies, respectively.
Varying responses of PML-RARA with different mutations to arsenic treatment validated in normal CD34+ HSCs and a human leukemia cell line
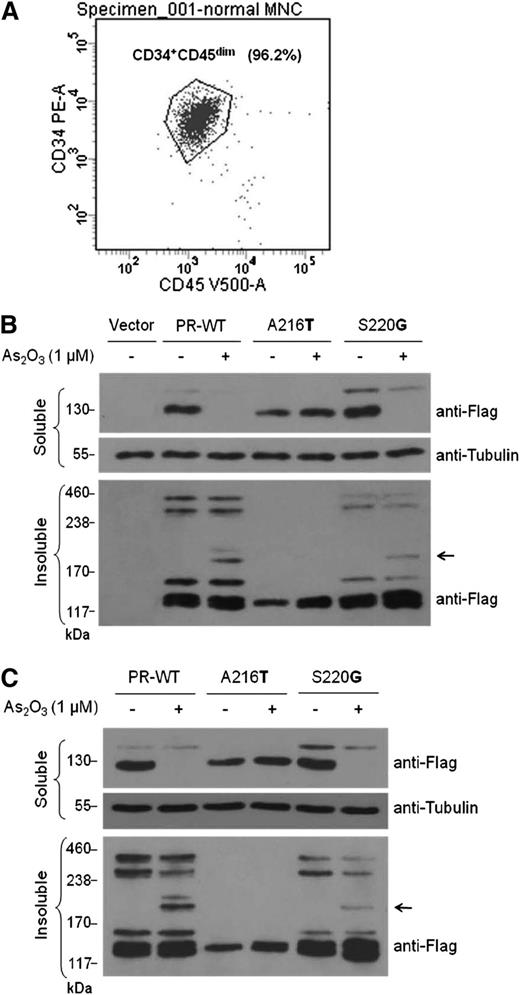
Next, different responses of PML-RARA mutants to arsenic treatment were confirmed in the hematopoietic context. Normal human CD34+ HSCs and human leukemiaU937cells were transduced with lentiviral constructs expressing wild-type PML-RARA (PR-WT), a representative arsenic-resistant mutant (A216T), or a representative nonresistant mutant (S220G). Consistent with the observations from HeLa cells, exposure to As2O3 triggered degradation of the total protein of PR-WT and S220G mutant in both CD34+ HSCs (Figure 5A-B) and U937 cells (Figure 5C), accompanied by increased multimerization in their detergent-insoluble fractions. Again, the A216T mutant exhibited a clear resistance to As2O3 treatment in the same conditions. These findings confirm the functional differences in the identified PML-RARA mutations in response to arsenic treatment in normal and malignant hematopoietic cells.
Varying responses of PML-RARA mutants to arsenic treatment were validated in hematopoietic cells. (A) Flow cytometry analysis of purified normal human CD34+ hematopoietic stem cells (HSCs). (B) Lentiviral-mediated delivery of control vector, wild-type PML-RARA (PR-WT), an arsenic-resistant mutant (A216T), or a nonresistant mutant (S220G) was performed in CD34+ HSCs. (C) U937 cells were transduced with lentiviral constructs expressing PR-WT, A216T, or S220G mutants. Infected cells were treated with or without 1 μM of As2O3 for 16 hours. Protein levels in the soluble and insoluble fractions from whole-cell lysates were detected by immunoblotting using the indicated antibodies.
Varying responses of PML-RARA mutants to arsenic treatment were validated in hematopoietic cells. (A) Flow cytometry analysis of purified normal human CD34+ hematopoietic stem cells (HSCs). (B) Lentiviral-mediated delivery of control vector, wild-type PML-RARA (PR-WT), an arsenic-resistant mutant (A216T), or a nonresistant mutant (S220G) was performed in CD34+ HSCs. (C) U937 cells were transduced with lentiviral constructs expressing PR-WT, A216T, or S220G mutants. Infected cells were treated with or without 1 μM of As2O3 for 16 hours. Protein levels in the soluble and insoluble fractions from whole-cell lysates were detected by immunoblotting using the indicated antibodies.
Arsenic resistance of PML-RARA with A216V, S214L, or A216T mutation overcome by either a high dose of As2O3 or combined As2O3-ATRA treatment
Upon ATRA resistance, the use of As2O3 yields a preferable outcome; however, no successful strategy to overcome As2O3 resistance has been developed. Given the observation that 1 μM of As2O3 induces the degradation of PR-L mutants with L217F or S220G, we addressed whether a higher concentration of arsenic could overcome the acquired resistance driven by other mutations (A216V, S214L, and A216T). First, the transduced U937 cells were treated with serial concentrations of As2O3 (1, 2.5, 5, 7.5, and 10 μM, respectively). Only 10 μM of As2O3 attenuated the expression of the drug-resistant mutant (A216T) and induced protein multimerization in the detergent-insoluble fraction, although the lower dosages had no influence in this context (Figure 6A). Consistently, treatment with 10 μM of As2O3 in transfected HeLa cells significantly decreased protein levels of both the long (Figure 6B) and short (Figure 6C) isoforms of PML-RARA with the indicated point mutations, and their associations with the nuclear matrix were enhanced. In the presence of HA-SUMO, administration of 10 μM of As2O3 for 1 hour induced significant degradation of PML-RARA mutants with A216V, S214L, or A216T (Figure 6D, top row). Accordingly, hyperSUMOylation was observed in the detergent-insoluble fractions of cells treated with a high dose (10 μM) of arsenic compared with a low dose (1 μM) of arsenic (Figure 6D, middle and bottom rows). These results demonstrated that increasing the arsenic concentration could reverse the acquired arsenic resistance driven by the indicated point mutations of PML-RARA.
A high concentration of arsenic or combination with ATRA was capable of reversing the resistance driven by acquired point mutations. (A) U937 cells were transduced with lentiviral constructs expressing A216T. Infected cells were treated without or with 1 μM, 2.5 μM, 5 μM, 7.5 μM, or 10 μM of As2O3 for 4 hours. Protein levels were detected using the indicated antibody. (B-C) 10 μM of As2O3 was used to treat HeLa cells transfected with A216V, S214L, or A216T mutants in the long (B) and short (C) isoforms of PML-RARA for 2 hours, respectively. Immunoblot analysis was performed as described before. (D) HeLa cells were treated with 1 μM or 10 μM of As2O3 for 1 hour after transfection with A216V, S214L, or A216T mutants in the presence of HA-SUMO. Protein levels in the soluble fraction (top row) and in the detergent-insoluble pellets (middle and bottom rows) were detected using anti-Flag, anti-Tubulin, and anti-HA primary antibodies. (E) U937 cells transduced with the lentiviral mutant (A216T) were treated with 1 μM or 5 μM of As2O3 alone, or together with 1 μM of ATRA, respectively, for 16 hours. (F) Single drug (1 μM, respectively) or the As2O3–ATRA combination was used to treat HeLa cells transfected with A216V, S214L, or A216T mutants for 16 hours. Immunoblot analysis was performed as described before. (G) A schematic representing various responses to As2O3 treatment of PML-RARA with different genetic mutations. Left: The regular dose of As2O3 binds to PML-RARA with L217F or S220G and triggers the degradation of proteins. In contrast, the regular dose of As2O3 might not bind to PML-RARA with A216V, S214L, or A216T and therefore drives resistance to arsenic therapy. Right: A high dose of As2O3 or the As2O3–ATRA combination could induce degradation of PML-RARA with A216V, S214L, or A216T, suggesting that the mutation-driven resistance is at least partly overcome.
A high concentration of arsenic or combination with ATRA was capable of reversing the resistance driven by acquired point mutations. (A) U937 cells were transduced with lentiviral constructs expressing A216T. Infected cells were treated without or with 1 μM, 2.5 μM, 5 μM, 7.5 μM, or 10 μM of As2O3 for 4 hours. Protein levels were detected using the indicated antibody. (B-C) 10 μM of As2O3 was used to treat HeLa cells transfected with A216V, S214L, or A216T mutants in the long (B) and short (C) isoforms of PML-RARA for 2 hours, respectively. Immunoblot analysis was performed as described before. (D) HeLa cells were treated with 1 μM or 10 μM of As2O3 for 1 hour after transfection with A216V, S214L, or A216T mutants in the presence of HA-SUMO. Protein levels in the soluble fraction (top row) and in the detergent-insoluble pellets (middle and bottom rows) were detected using anti-Flag, anti-Tubulin, and anti-HA primary antibodies. (E) U937 cells transduced with the lentiviral mutant (A216T) were treated with 1 μM or 5 μM of As2O3 alone, or together with 1 μM of ATRA, respectively, for 16 hours. (F) Single drug (1 μM, respectively) or the As2O3–ATRA combination was used to treat HeLa cells transfected with A216V, S214L, or A216T mutants for 16 hours. Immunoblot analysis was performed as described before. (G) A schematic representing various responses to As2O3 treatment of PML-RARA with different genetic mutations. Left: The regular dose of As2O3 binds to PML-RARA with L217F or S220G and triggers the degradation of proteins. In contrast, the regular dose of As2O3 might not bind to PML-RARA with A216V, S214L, or A216T and therefore drives resistance to arsenic therapy. Right: A high dose of As2O3 or the As2O3–ATRA combination could induce degradation of PML-RARA with A216V, S214L, or A216T, suggesting that the mutation-driven resistance is at least partly overcome.
Previous studies have reported that the combination with ATRA worked better than As2O3 alone for APL. Here, combined treatment with As2O3 and ATRA was applied in U937 cells transduced with an arsenic-resistant mutant. As shown in Figure 6E, either 1 μM or 5 μM of As2O3 together with 1 μM of ATRA induced degradation of the total protein of the A216T mutant, accompanied by increased multimerization in their detergent-insoluble fractions. Moreover, As2O3 resistance driven by A216V, S214L, or A216T was similarly overcome by the As2O3–ATRA combination in HeLa cells (Figure 6F). Taken together, our results demonstrated that the combination of As2O3 and ATRA might overcome the acquired arsenic resistance driven by the mutations in the PML B2 domain of the PML-RARA oncoprotein.
Differential responses to arsenic reinduction treatment observed in relapsed APL patients carrying various genetic mutations
Finally, we performed a retrospective analysis to validate whether the varying degrees of arsenic resistance triggered by different PML-RARA mutations are conserved in APL patients. As shown in Table 1, patients 1 and 2, who both had the A216V mutation, did not achieve remission after reinduction treatment with arsenic. Of note, patient 2 had a molecular relapse with a significantly positive expression (38.6%) of PML-RARA transcripts in bone marrow. Patient 3, who also carried the A216V mutation at relapse, abandoned the ongoing reinduction therapy because of rapid disease progression and died soon thereafter. Although the morphologic characteristics and PML-RARA transcript levels of this patient were not available after reinduction, attenuated arsenic efficacy could be a reasonable explanation for the deteriorative outcome. Similarly, apparent resistance to arsenic-combined treatment was observed in patient 4, who had both the S214L and A216T mutations, and the other 3 patients (5-7) who had the A216T mutation. The percentage of APL cells in bone marrow from all of these patients was ≥15% after arsenic reinduction treatment. As indicated in Table 1, patient 5 experienced a molecular relapse as well, with PML-RARA transcript levels increased from 0% to 14.3%. In brief, the strong arsenic resistance driven by A216V, S214L, or A216T mutations observed in vitro was mirrored in the clinical setting.
Varying responses to arsenic reinduction treatment observed in patients with relapsed APL and carrying genetic mutations within PML-RARA
| Patient No. . | Acquired mutations . | APL cells in bone marrow . | |
|---|---|---|---|
| Before reinduction treatment . | After reinduction treatment . | ||
| 1 | A216V | 48% | 20% |
| 2 | A216V | 2%*a | 43% |
| 3 | A216V | 10% | UA |
| 4 | S214L+A216T | 48% | 52% |
| 5 | A216T | 2%*b | 15% |
| 6 | A216T | 66% | 46% |
| 7 | A216T | 44% | 85% |
| 8 | L217F | 96% | 0 |
| 9 | S220G | 69% | 11% |
| Patient No. . | Acquired mutations . | APL cells in bone marrow . | |
|---|---|---|---|
| Before reinduction treatment . | After reinduction treatment . | ||
| 1 | A216V | 48% | 20% |
| 2 | A216V | 2%*a | 43% |
| 3 | A216V | 10% | UA |
| 4 | S214L+A216T | 48% | 52% |
| 5 | A216T | 2%*b | 15% |
| 6 | A216T | 66% | 46% |
| 7 | A216T | 44% | 85% |
| 8 | L217F | 96% | 0 |
| 9 | S220G | 69% | 11% |
UA, unavailable (as described in the text).
The percentage of APL cells in bone marrow from included patients were examined before and after reinduction treatment with arsenic.
Relapse occurred at the molecular level. The expression levels of PML-RARA transcripts are represented by “a” (39.8%) and “b” (14.3%) respectively.
Conversely, patient 8, who had the L217F mutation, achieved a complete remission at both the morphologic and molecular levels after reinduction therapy with IV As2O3. This patient received oral As4S4 as one of the induction and consolidation regimens before relapse. This case is the only one among the 9 relapsed APL patients with acquired mutations who survived during the study, and the patient is still alive to date. This observation validates the findings from in vitro experiments showing that PML-RARA mutants with L217F were totally degraded upon As2O3 exposure.
Patient 9 had the S220G mutation, and morphologic analysis showed a partial response to reinduction treatment, with a reduction in the percentage of APL cells from 69% to 11%; however, the level quickly returned to 48%, and the patient died soon thereafter. Consistent with the findings from in vitro experiments as shown before, our retrospective analysis showed temporary moderate drug resistance in a clinical model with the S220G mutation. One of the possible explanations could be that this case carried both the PML and RARA mutations, which also affected the efficacy of ATRA during reinduction therapy.
Discussion
PML-RARA multimerization and arsenic trioxide binding, followed by protein hyperSUMOylation and degradation, constitute the molecular mechanism of arsenic-induced treatment of APL.14,15 Genetic mutations in the PML moiety of PML-RARA are present in quite a few patients with relapsed/refractory APL. The fact that these acquired point mutations attenuate the efficiency of arsenic in an ex vivo model poses a big challenge for APL management in the clinic. In addition to providing more evidence to elucidate the causal role of acquired mutations within PML-RARA in mediating arsenic resistance during APL therapy, we also report a functional difference among the identified point mutations.
Of the 5 identified point mutations in the same domain of PML-RARA, our current findings indicated that the A216V, S214L, and A216T mutations resulted in strong resistance to arsenic treatment in both the experimental and clinical models. By contrast, L217F and S220G mutations may drive weak resistance to arsenic (summarized in Figure 6G). The cause of the varying responsiveness of different PML-RARA mutants is worth investigating. The As2O3-binding cysteine residues are located inside the PML-B2 domain and are required for As2O3-triggered degradation of PML/PML-RARA.5,6 Given that the identified mutational region (S214-S220) is immediately adjacent to C212/213, different point mutations might have different influences on the protein conformation and therefore disturb the binding of arsenic with cysteine residues in the PML region of PML-RARA.
The association of genetic mutations with drug resistance is more complicated in the clinical context. First, in addition to the acquired mutations identified in the PML moiety of PML-RARA, prolonged ATRA treatment can be associated with resistance linked to mutations inside the ligand-binding domain of the RARA moiety or by inducing aberrant transcription repression complexes.16-18 Different PML mutations combining mutant RARA might trigger drug resistance to varying degrees. Second, another study recently reported that the acquired A216V mutation was found in the PML allele, which was not rearranged, from an APL patient with multiple relapses after arsenic-combination treatment.19 However, this mutation was not present in the PML moiety of the PML-RARA fusion gene, suggesting the dual action of arsenic trioxide on both PML-RARA and PML. Third, it is important to know whether arsenic-resistant mutations exist at the time of diagnosis. Of 9 patients included in the present work, one was evaluated at diagnosis and no mutation was found, as described previously.8 We also evaluated 3 other patients before they exhibited arsenic resistance and did not find mutations in PML-RARA.8 Although these results support the notion that arsenic-resistant mutations were acquired in the course of treatment, we cannot rule out the possibility that small, highly diluted clones were present, though undetectable, at presentation because of limited sensitivity of the assay. Future investigations should include sequencing analysis to identify PML mutations at the time of diagnosis.
The A216V mutation of PML-RARA has been identified by both Goto et al7 and our group in APL patients with arsenic resistance. In an in vitro experimental system, we found that the A216V mutant was resistant to low concentrations (1 μM or 5 μM) but definitely responded to a high dose (10 μM) of As2O3, which we confirmed with both the long and short isoforms of PML-RARA. This finding contrasts with the previous report by Goto et al showing that the expression of the short isoform of PML-RARA with the A216V mutation was not inhibited by 10 μM of As2O3 in HeLa cells. Because only a single concentration was used in that work, this inconsistent finding could be explained by differences in assay sensitivity and/or transfection efficiency between the 2 independent groups.
Arsenic is generally toxic to humans and can induce potentially lethal damage to multiple organs20-22 ; however, its reputation as a therapeutic agent has increased in the last 2 decades.23-27 The routine dosage of arsenic trioxide used in clinical treatment is approximately 0.08 to 0.16 mg/kg per day.4,25 For some patients with relapsed APL, however, the conventional dosage of As2O3 fails to eradicate malignant cells. We previously showed that plasma arsenic concentrations in APL patients during induction therapy with oral As4S4 were lower than those receiving intravenous As2O3.4 Our unpublished data also show that the intracellular concentrations of RIF (formula with As4S4 as the principle ingredient) during therapy were significantly lower than those of As2O3. Interestingly, a similar efficacy between As4S4 and As2O3 treatment was observed in both studies. Therefore, it is worth trying to increase the oral dose of As4S4 in relapsed APL patients, which could elevate the intracellular arsenic concentration. In addition, Wang et al28 reported that the adjuvant components facilitate the transportation of As4S4 into APL cells and enhance PML-RARA degradation, which opens a new window to increasing the cellular concentration of arsenic for overcoming drug resistance.
Previous studies have demonstrated that the combination of As2O3 and ATRA is effective treatment of newly diagnosed APL.29-31 In the current work, we found that As2O3 (1 μM) plus ATRA (1 μM) could overcome arsenic resistance in the cultured cell lines. Although the mechanism underlying this synergistic efficacy awaits further investigation, our findings suggest the possibility that a combined regimen might work for relapsed APL resistant to As2O3. However, we did not observe a response to the combined treatment of patients (1-4; Table 1) with arsenic-resistant mutations at APL relapse. These patients were administrated 25 mg/m2 of ATRA per day during the reinduction therapy. A previous study reported that, for patients at APL relapse, the peak plasma concentration of ATRA was about 0.12 μg/mL (0.4 μM) after treatment with 45 mg/m2 per day.32 Thus, the concentrations of ATRA achieved in patients’ plasma in the current study might be much lower than the concentration used for transfected cell lines, resulting in the inconsistent responses between in vivo and in vitro. Another explanation might be that the case number of the present retrospective study is too limited, leading to an inability to observe a beneficial effect of ATRA plus As2O3 on relapsed APL.
In summary, APL that proves resistant to As2O3 and/or ATRA is a challenging problem in the clinic. Our results reinforce the causal role of genetic mutations found in the PML part of the PML-RARA fusion gene in mediating arsenic resistance during APL therapy. We also propose the novel insight that acquired point mutations could exhibit varying responses to arsenic treatment. Detailed genomic sequencing analysis may help in predicting the prognosis and selecting more effective regimens to treat APL. Furthermore, we demonstrated that a high concentration of As2O3 or As2O3 in combination with ATRA could overcome the acquired arsenic resistance triggered by the indicated point mutations in PML-RARA. Our findings not only elucidate the association of PML-RARA mutations with arsenic efficacy but also pave the way for future research to improve outcomes for patients with relapsed/refractory APL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Martin S. Tallman for reading the manuscript.
This work is supported by a grant from the Beijing Municipal Science and Technology Program (grant Z141100000214011).
Authorship
Contribution: J.L. designed and performed the research, analyzed data, and wrote the manuscript; H.-H.Z. performed the research and analyzed data; H.J. and Q.J. collected and interpreted data; and X.-J.H. designed and supervised research and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiao-Jun Huang, 11 Xizhimen South St, Beijing 100044, China; e-mail: xjhrm@medmail.com.cn.
References
Author notes
J.L. and H.-H.Z. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal