Abstract
Our understanding of the pathogenesis and heterogeneity of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) has been dramatically enhanced by recent attempts to profile molecular features of these lymphomas. In this article, we discuss ways in which testing for molecular features may impact DLBCL and FL management if clinical trials are designed to incorporate such tests. Specifically, we discuss how distinguishing lymphomas on the basis of cell-of-origin subtypes or the presence of other molecular features is prognostically and therapeutically significant. Conversely, we discuss how the molecular similarities of DLBCL and FL have provided insight into the potential of both DLBCL and FL cases to respond to agents targeting alterations they have in common. Through these examples, we demonstrate how the translation of our understanding of cancer biology into improvements in patient outcomes depends on analyzing the molecular correlates of treatment outcomes in clinical trials and in routinely treated patients.
Introduction
Molecular characteristics of cancers have increasingly been used to guide drug development, predict patient outcomes, and inform treatment decisions. In particular, recent insights into the molecular heterogeneity of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) are driving a paradigm shift toward more personalized therapies for these lymphomas. This article considers how our understanding of cancer biology can be used to develop and test new therapies, and highlights the ways in which new biomarkers, including mutations and gene expression signatures, could become routinely used in the clinic. Using these examples, we examine how our capacity to identify and characterize biomarkers depends on the acquisition, organization, and accessibility of matched clinical and molecular data. Overall, we present the viewpoint that the collection and evaluation of molecular signatures in clinical samples is key to improving patient outcomes.
The cellular origin of DLBCL and FL
DLBCL is an aggressive lymphoma that can be subclassified on the basis of histologic, clinical, immunohistochemical, and molecular features.1 Gene expression profiling has identified subtypes of DLBCL whose cells of origin are thought to differ. Germinal center B-cell (GCB) DLBCL has a gene expression signature characteristic of germinal center (GC) B cells.2 GC B cells are produced from mature B cells in lymphoid organs that have been activated by interactions with an antigen and T-helper cells.3 Differentiation into GC B cells activates somatic hypermutation, a process that generates diversity in the immunoglobulin gene regions. GC B cells displaying immunoglobulins with high affinity for antigen receive survival signals and may differentiate into antibody-producing plasma cells or memory B cells. In contrast, activated B-cell (ABC) DLBCL has a gene expression signature similar to that of plasma cells and is thus thought to arise from post-GC B cells undergoing plasmacytic differentiation.4 A third cell-of-origin subtype of DLBCL, primary mediastinal B-cell lymphoma, is thought to originate from thymic B cells5 but, for brevity, is not discussed further here.
It is notable that the 5-year survival rate for patients treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone is ∼80% for those with GCB DLBCL but only ∼50% for those with ABC DLBCL.6 Cell-of-origin subtype is determined by many pathologists using a classifier based on immunohistochemical staining for CD10, BCL6, and IRF4 (also called MUM1) proteins.1,7 However, immunohistochemical classifiers are less accurate than gene expression–based methods for cell-of-origin subtyping.8 Gene expression–based methods for cell-of-origin classification that are suitable for standard practice have recently been developed9-11 and may soon be more commonly used than the immunohistochemical classifiers.
In contrast to the aggressive nature of DLBCL, FL is an indolent disease. Despite this difference in clinical behavior, FL, like GCB DLBCL, develops from GC B cells.2,12 Perhaps in part because of this similarity in cell of origin, many of the “driver” genetic alterations of DLBCL (ie, alterations that provide a selective advantage and thus contribute to cancer development) are also drivers of FL. Examples of how therapies that are designed to target such alterations may be effective against both DLBCL and FL are provided throughout this article (summarized in Table 1 13-33 and Figure 1). Moreover, several markers of prognostic significance are relevant to both DLBCL and FL management. For instance, the presence of IRF4 protein is an independent prognostic factor for shorter progression-free survival in FL34 and is characteristic of ABC DLBCL, the DLBCL subtype with poorer prognosis.35
Targeted therapies in development for DLBCL and FL
| Molecular feature . | Driver of DLBCL or FL? . | Targeted therapeutics in development . | Reference . | Clinical trial number . |
|---|---|---|---|---|
| BCL2 expression | ABC and GCB DLBCL and FL | ABT-199, navitoclax, IMC-48 | 13-15 | NCT01328626, NCT00788684 |
| MYC and either BCL2 or BCL6 expression (“double hit”) | ABC and GCB DLBCL | BET inhibitors (eg, OTX015), TMPyP4 | 16-19 | NCT01713582 |
| CXCR4 expression | ABC and GCB DLBCL and FL | BKT140, AMD3100, BKM120 | 20-23 | |
| PTEN inactivation | GCB DLBCL and FL; uncommon in ABC DLBCL | PI3K inhibitors (eg, BKM120), mTOR inhibitors (eg, everolimus), AKT inhibitors | 23, 24 | NCT01719250, NCT0204954, NCT01693614, NCT00869999 |
| EZH2 mutation | GCB DLBCL and FL | EPZ-6438, GSK2816126 | 25, 26 | NCT01897571, NCT02082977 |
| Loss of EPHA7 expression | ABC and GCB DLBCL and FL | Recombinant EPHA7 | 27 | |
| Constitutive activation of NF-κB signaling | ABC DLBCL and FL | Bortezomib | 28, 29 | NCT00057902, NCT00715208 |
| Constitutive activation of B-cell receptor signaling | ABC DLBCL and FL | Ibrutinib | 30, 31 | NCT01804686, NCT01569750 |
| Molecular feature . | Driver of DLBCL or FL? . | Targeted therapeutics in development . | Reference . | Clinical trial number . |
|---|---|---|---|---|
| BCL2 expression | ABC and GCB DLBCL and FL | ABT-199, navitoclax, IMC-48 | 13-15 | NCT01328626, NCT00788684 |
| MYC and either BCL2 or BCL6 expression (“double hit”) | ABC and GCB DLBCL | BET inhibitors (eg, OTX015), TMPyP4 | 16-19 | NCT01713582 |
| CXCR4 expression | ABC and GCB DLBCL and FL | BKT140, AMD3100, BKM120 | 20-23 | |
| PTEN inactivation | GCB DLBCL and FL; uncommon in ABC DLBCL | PI3K inhibitors (eg, BKM120), mTOR inhibitors (eg, everolimus), AKT inhibitors | 23, 24 | NCT01719250, NCT0204954, NCT01693614, NCT00869999 |
| EZH2 mutation | GCB DLBCL and FL | EPZ-6438, GSK2816126 | 25, 26 | NCT01897571, NCT02082977 |
| Loss of EPHA7 expression | ABC and GCB DLBCL and FL | Recombinant EPHA7 | 27 | |
| Constitutive activation of NF-κB signaling | ABC DLBCL and FL | Bortezomib | 28, 29 | NCT00057902, NCT00715208 |
| Constitutive activation of B-cell receptor signaling | ABC DLBCL and FL | Ibrutinib | 30, 31 | NCT01804686, NCT01569750 |
Listed are only those agents discussed in this article. More comprehensive listings of targeted therapeutics in development can be found in recent reviews.32,33
AKT, protein kinase B; BET, bromodomain and extraterminal domain; mTOR, mechanistic target of rapamycin; NF, nuclear factor; PI3K, phosphatidylinositol 3-kinase.
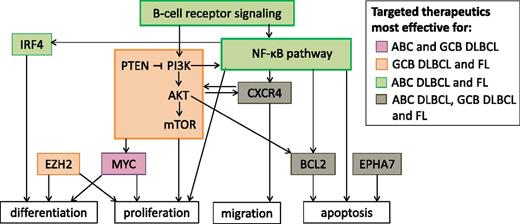
Signaling pathways and proteins that are targets for new DLBCL and FL therapeutics. Only pathways and proteins discussed in this article are included in this figure. Signaling pathways and proteins are colored according to whether agents targeting them are expected to be more effective against ABC DLBCL, GCB DLBCL, or FL. Arrows indicate interactions between components.
Signaling pathways and proteins that are targets for new DLBCL and FL therapeutics. Only pathways and proteins discussed in this article are included in this figure. Signaling pathways and proteins are colored according to whether agents targeting them are expected to be more effective against ABC DLBCL, GCB DLBCL, or FL. Arrows indicate interactions between components.
Genetic alterations driving GCB DLBCL and FL
Focusing first on alterations in common between GCB DLBCL and FL, one of particular clinical interest is the t(14;18)(q32;q21) translocation. This translocation is present in up to 85% of FL cases36 and 34% of GCB DLBCL cases but is rare in ABC DLBCL.37 Constitutively high levels of the antiapoptotic protein BCL2 are produced as a result of this translocation.38,39 Although these statistics alone may lead one to infer that ABC DLBCL, unlike GCB DLBCL and FL, is not driven by BCL2 overexpression, analyses of BCL2 abundance do not support this conclusion. Specifically, ∼60% of ABC DLBCL exhibited high levels of BCL2,40 typically as a result of BCL2 copy number amplification (present in 46% of ABC DLBCL) or alterations activating NF-κB signaling upstream of BCL2 expression.41 Thus, BCL2 overexpression may be a driver of both subtypes, and agents targeting BCL2 may be effective against both subtypes. This example highlights how multiple mechanisms of dysregulation must be considered when evaluating whether a particular protein may contribute to disease pathogenesis.
Enhancing the attractiveness of BCL2 as a drug target is evidence that when a BCL2 alteration is detected in a patient, that alteration tends to be present in all samples of that patient’s lymphoma.42,43 Agents targeting BCL2 may thus be effective against most, if not all, of the tumor burden in the majority of patients with BCL2 overexpression. The BCL2 inhibitor ABT-199 produced responses in 3 of 9 DLBCL cases and in 3 of 11 FL cases in phase 1 trials.13 Another BCL2 inhibitor, navitoclax, has also been tested in phase 1 trials for FL and produced responses in 9 of 12 patients.14 However, it is unclear whether treatment response correlated with the presence of genetic alterations impacting BCL2 expression. Identifying such a correlation would be necessary to develop these alterations as biomarkers predictive of response to BCL2 inhibitors.
Although BCL2 protein levels were not prognostic indicators in FL,44 BCL2 mutations, which occur in ∼12% of FL, were an independent risk factor for poor survival.45 In DLBCL, poor prognosis is associated with a combination of translocations activating MYC and either BCL2 or, less commonly, another oncogene such as BCL6.46 Such “double hits” are independently associated with shorter progression-free and overall survival.47-49 MYC promotes DLBCL proliferation but can also sensitize cells to apoptotic signals.50 Synergistic effects of increased BCL2 or BCL6 abundance may thus arise from the antiapoptotic effects of those proteins,50 although the precise mechanism driving the aggressive nature of these lymphomas remains to be demonstrated.
Some studies more broadly defined double-hit lymphomas as those in which the abundance of MYC and either BCL2 or BCL6 is increased by any mechanism.40,51,52 This expanded definition raises the proportion of DLBCL considered to be double hit (from 5%-10% to 30%-40%) and allows greater inclusion of ABC DLBCL samples.53 Significant, albeit smaller, differences in outcome continued to be observed when using this more inclusive definition.40,51,52 Testing of all DLBCL cases for MYC and BCL2 abundance using immunohistochemistry has therefore been recommended.53
Chemotherapy regimens more aggressive than rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (eg, dose-adjusted rituximab plus etoposide, prednisone, vincristine, cyclophophamide, and doxorubicin) have been recommended for double-hit DLBCL53 on the basis of retrospective evidence that such therapy improves survival.49 However, prospective studies have not yet been completed. Other agents that may prove effective against double-hit DLBCL are (1) small molecules that stabilize repressive secondary structures of the BCL2 or MYC promoters15,16,54 and (2) inhibitors of BET proteins, which are readers of histone acetylation marks that promote transcriptional activation.55 BET inhibitors can decrease the expression of MYC and other oncogenes17,18 and are currently in phase 1 clinical trials for DLBCL and FL.19 Of interest, studies using DLBCL cell lines identified a gene expression signature associated with response to the BET inhibitor OTX015. Moreover, OTX015 had apoptotic rather than cytostatic effects in a genetically defined subgroup of DLBCL cell lines.19 It would be of clinical interest to determine whether these molecular signatures can predict treatment response in patients.
Of note, the combination of BCL2 translocation and CXCR4 expression was recently found to be associated with outcomes as poor as those for double-hit DLBCL.20 Independent of other factors, CXCR4 expression was associated with poorer progression-free survival in GCB DLBCL, but not in ABC DLBCL.20,21 CXCR4 is a chemokine receptor known to promote cancer cell migration to tissues such as the bone marrow and lymph nodes.56,57 The CXCR4 inhibitor BKT140 decreased the growth of DLBCL cell lines, although, curiously, CXCR4 expression levels did not correlate with the degree of growth inhibition.20
CXCR4 signaling is also relevant to FL. Specifically, some FL cells resisted chemotherapy through CXCR4-dependent interactions with follicular dendritic cells.22 Moreover, treatment with the CXCR4 inhibitor AMD3100 eliminated the capacity of drug-resistant FL cells to form tumors in mice.22 Cross talk with follicular dendritic cells can also be reduced by decreasing levels of the CXCR4 ligand CXCL12, as can be done using the PI3K inhibitor BKM120.23 BKM120 reduced FL cell proliferation in mouse xenografts,23 perhaps in part through effects on interactions with follicular dendritic cells and in part through disruption of the survival signals that PI3K activity would otherwise convey.58 Clinical trials of BKM120 for FL are ongoing (NCT01719250, NCT0204954, and NCT01693614).
PI3K signaling appears constitutively activated in a subset of FLs and may therefore contribute to FL development.59 Constitutive PI3K signaling is also thought to contribute to GCB DLBCL because PTEN, a repressor of PI3K, is inactivated in 55% of GCB DLBCL.60 PTEN inactivation is less common in ABC DLBCL, occurring in only 14% of cases.60 Consequently, inhibitors of proteins that interact with PTEN signaling (eg, PI3K and mTOR) are expected to be more effective against GCB DLBCL compared to ABC DLBCL.32 mTOR inhibitors have shown particular efficacy, with everolimus in combination with rituximab producing a response in 9 of 24 patients in a phase 2 study on relapsed/refractory DLBCL.24 However, response to everolimus did not correlate with mTOR/PI3K pathway activation.24 Such a correlation may have been masked by variability in the time between sample collection and patient treatment and by the sensitivity of protein phosphorylation to variation in the time between biopsy and fixation. This example highlights the necessity of careful forethought regarding the manner in which tissue for molecular analysis is obtained if one aims to identify biomarkers. The accuracy of phosphoprotein analyses can be increased by the use of phosphatase and kinase inhibitors61 and a shortened fixation protocol.62
Mutations in the H3K27 methyltransferase gene EZH2 are also common in GCB DLBCL (22%-24% of cases) and FL (7% of cases), but not in ABC DLBCL (<4% of cases).25,63,64 EZH2 mutations may drive GCB DLBCL and FL development by promoting B-cell proliferation and by impeding plasmacytic differentiation.26 ABC DLBCL may not be effectively driven by EZH2 mutations because the cell of origin of ABC DLBCL has progressed pass the developmental stage during which EZH2 inhibits plasmacytic differentiation. Phase 1 clinical trials of EZH2 inhibitors on DLBCL and FL are in progress (NCT01897571 and NCT02082977). These trials distinguish samples with EZH2 mutations from those without, allowing an assessment of whether EZH2 mutations are predictive of response.
EZH2, PI3K, mTOR, CXCR4, BCL2, and MYC proteins were attractive targets for therapies, in part because they tended to be activated rather than inactivated in lymphoma. Thus, drug development involved the development of inhibitors rather than the more complex task of restoring protein activity. Nonetheless, protein replacement therapy has shown promise for some secreted proteins that may be synthesized in vitro. For instance, EPHA7 is a small secreted protein that normally inhibits oncogenic signaling by binding EPHA2 receptors.27 EPHA7 expression is lost in 72% of FL and 15% of DLBCL,27,65,66 allowing oncogenic signaling to proceed uninhibited. When administered intravenously, recombinant EPHA7 conjugated to CD20 antibody induced complete responses in EPHA7-negative lymphoma xenographs.27 Clinical trials with this agent are thus anticipated67 and may investigate EPHA7 loss as a predictive biomarker for response to this treatment.
There also exist numerous candidate driver alterations found much more frequently in DLBCL than in FL, or vice versa. The identification of differences in driver mutations between FL and DLBCL has been key to improving our understanding of how FL can transform into secondary DLBCL, a cancer that is histologically and clinically similar to de novo DLBCL.68,69 Such transformation occurs at a cumulative rate of 2% to 3% a year.70,71 Identifying biomarkers that predict risk of transformation or that can facilitate the early diagnosis of transformation may aid in management.72 Of interest, chronic lymphocytic leukemia can also transform into secondary DLBCL. Unlike secondary DLBCL arising from FL, secondary DLBCL arising from chronic lymphocytic leukemia does not respond to treatment with the immune modulator lenalidomide.73 Thus, techniques for determining the histologic origin of DLBCL in cases where it may be unclear from patient history may be of clinical utility. Similarly, emerging differences in the underlying biology of de novo and secondary DLBCL74 may prove prognostically or therapeutically significant, warranting the development of biomarkers to molecularly distinguish de novo and secondary DLBCL.
Genetic alterations driving ABC DLBCL
Several oncogenic changes common in ABC, but not GCB, DLBCL are targets for therapies that may become routinely used only for the ABC subtype. For instance, ABC DLBCL is characterized by mutations constitutively activating NF-κB signaling. NF-κB signaling inhibits apoptosis75 and is required for survival of ABC, but not GCB, DLBCL cells.28,76 This differential requirement for NF-κB may explain why the proteasome and NF-κB pathway inhibitor bortezomib produced greater response rates in ABC DLBCL than in GCB DLBCL (10/12 vs 2/15 cases) when used in combination with doxorubicin-based chemotherapy in a phase 2 study.77 Alterations affecting B-cell receptor signaling are also more common in ABC DLBCL than in GCB DLBCL, in part because NF-κB activity is activated downstream of B-cell receptor signaling.75 Consistent with this biology, agents such as ibrutinib that inhibit B-cell receptor signaling have shown greater efficacy in ABC DLBCL than in GCB DLBCL (ie, response rates of 10/29 vs 1/20 cases).30 Ibrutinib has now entered a phase 3 clinical trial for non-GCB DLBCL78 in which tumor biopsy samples will be collected for biomarker evaluation at the time of disease progression (NCT01804686). The resulting biomarker data may be used to identify molecular features associated with treatment resistance.
Of interest, a retrospective analysis of clinical trial data found that the immunomodulatory drug lenalidomide also had greater activity against ABC DLBCL compared to GCB DLBCL (ie, 53% vs 9% overall response rate).79 Lenalidomide is now in a phase 3 clinical trial (NCT02285062) that distinguishes the ABC subtype using gene expression profiling rather than the less accurate8 immunohistochemical methods used in prior clinical trials.79 The cytotoxic effects of lenalidomide on DLBCL cells are due in part to a β-interferon response that results from the downregulation of IRF4.80 Of note, a study of xenografted ABC DLBCL cells found that the cytotoxic effects of lenalidomide synergized with those of ibrutinib, perhaps because both agents decrease IRF4 expression.80 Phase 1b/2 clinical trials combining lenalidomide and ibrutinib treatment of relapsed/refractory DLBCL are ongoing (NCT02142049 and NCT02077166).
However, inhibitors of B-cell receptor signaling are not effective against lymphomas with activating mutations downstream of the therapeutic target. For instance, CARD11 acts downstream of the protein inhibited by ibrutinib; thus, lymphomas with mutations activating CARD11 (∼10% of ABC DLBCL cases81 ) do not respond to ibrutinib.82,83 Screening for such mutations that confer resistance could help inform the choice of therapy. Moreover, it may become possible to screen for a molecular signature correlated more closely than cell-of-origin subtype with response to inhibitors of B-cell receptor signaling.83 However, such a signature remains to be identified.
Of interest, FLs have responded to some of the agents that are more effective against ABC DLBCL than GCB DLBCL. For instance, a response of FL to ibrutinib was observed in phase 1 clinical trials,31,84 and lenalidomide and bortezomib produced response rates of 76% to 98% in phase 2 clinical trials in FL.29,85-87 Responses to these agents were not surprising because recurrent mutations have been identified in FL in components of the NF-κB and B-cell receptor signaling pathways.88 Thus, despite their slightly different cells of origin, some cases of FL and ABC DLBCL appear to be driven by dysregulation of the same pathways. Predictive biomarkers identified in DLBCL clinical trials may thus inform the design and analysis of FL clinical trials, and vice versa.
Conclusions
The molecular similarities of DLBCL and FL have provided insight into shared aspects of their pathogenesis and the potential of DLBCL and FL cases to respond to agents targeting alterations they have in common. Conversely, distinguishing lymphomas on the basis of cell-of-origin subtypes or the presence of other molecular features has proven biologically, prognostically, and therapeutically significant. However, the use of distinct treatment regimens for molecularly defined subtypes can become routine practice only if phase 3 clinical trials are designed in a manner that distinguishes the relevant subtypes. Neglecting to characterize predictive biomarkers decreases the efficiency with which treatment resources can be used and puts patients at risk of either not receiving a therapy that would have been effective or suffering side effects from an unnecessary treatment. Moreover, new therapies highly effective against cancers with certain molecular features may not be recognized as such if the relevant molecular data are not collected from clinical trials. Potentially ground-breaking new therapeutics may thus be overlooked.
Although it is logical to expect that an inhibitor of a particular protein will be most effective against lymphomas that are driven by the increased activity of that protein, identifying such cases may not be straightforward. Treatment response can be affected not only by multiple types of lesions affecting a given target but also by alterations affecting other components of the pathways in which that target acts. The best predictors of treatment response, therefore, may not be the presence or absence of a single alteration, but rather signatures integrating multiple factors. Such predictive signatures may be identified by analyzing molecular correlates of treatment outcomes in routinely treated patients and in clinical trials. However, obtaining and organizing such data will require significant forethought, interdisciplinary collaboration, and supportive infrastructure. Moreover, the incorporation of new biomarkers into clinical practice will require pathologists and oncologists to understand the utility and limitations of such tests. Overall, the utility of molecular markers does not end at obtaining a better understanding of cancer biology or at identifying new drug targets; the collection and evaluation of molecular signatures in clinical samples is needed for the continued improvement of patient outcomes.
Acknowledgments
J.R.P. is supported by a Canadian Institutes of Health Research Vanier Canada Graduate Scholarship, a Scriver MD/PhD Scholarship, and a University of British Columbia Four Year Fellowship. M.A.M. is the University of British Columbia Canada Research Chair in Genome Science. The BC Cancer Agency Genome Sciences Centre gratefully acknowledges funding support from the BC Cancer Foundation (NSA10108), the Terry Fox Research Institute (award #1023), Genome Canada (award #4108), Genome British Columbia, and the Leukemia and Lymphoma Society of Canada. The authors also thank John Auston and family for their generous support.
Authorship
Contribution: J.R.P. and M.A.M. wrote and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marco A. Marra, Genome Sciences Centre, BC Cancer Agency, 675 West 10th Ave, Vancouver, BC, Canada V5Z 1L3; e-mail: mmarra@bcgsc.ca.