Key Points
Hyperactivation of the BMP-SMAD pathway blunts EPO-mediated hepcidin inhibition.
Lack of BMP-SMAD pathway inhibition by matriptase-2 abrogates the ERFE-mediated hepcidin suppression in response to EPO.
Abstract
Hepcidin, the main regulator of iron homeostasis, is repressed when erythropoiesis is acutely stimulated by erythropoietin (EPO) to favor iron supply to maturing erythroblasts. Erythroferrone (ERFE) has been identified as the erythroid regulator that inhibits hepcidin in stress erythropoiesis. A powerful hepcidin inhibitor is the serine protease matriptase-2, encoded by TMPRSS6, whose mutations cause iron refractory iron deficiency anemia. Because this condition has inappropriately elevated hepcidin in the presence of high EPO levels, a role is suggested for matriptase-2 in EPO-mediated hepcidin repression. To investigate the relationship between EPO/ERFE and matriptase-2, we show that EPO injection induces Erfe messenger RNA expression but does not suppress hepcidin in Tmprss6 knockout (KO) mice. Similarly, wild-type (WT) animals, in which the bone morphogenetic protein–mothers against decapentaplegic homolog (Bmp-Smad) pathway is upregulated by iron treatment, fail to suppress hepcidin in response to EPO. To further investigate whether the high level of Bmp-Smad signaling of Tmprss6 KO mice counteracts hepcidin suppression by EPO, we generated double KO Bmp6-Tmprss6 KO mice. Despite having Bmp-Smad signaling and hepcidin levels that are similar to WT mice under basal conditions, double KO mice do not suppress hepcidin in response to EPO. However, pharmacologic downstream inhibition of the Bmp-Smad pathway by dorsomorphin, which targets the BMP receptors, improves the hepcidin responsiveness to EPO in Tmprss6 KO mice. We concluded that the function of matriptase-2 is dominant over that of ERFE and is essential in facilitating hepcidin suppression by attenuating the BMP-SMAD signaling.
Introduction
The production of red blood cells (RBCs) is a coordinated process that requires both the growth factor erythropoietin (EPO) and an adequate iron supply. EPO, produced by the kidney during hypoxia, stimulates the erythroid cells proliferation and differentiation.1 To acquire iron, essential for hemoglobin (Hb) synthesis, erythroid precursors release soluble factors that suppress the expression of the hepatic iron regulatory hormone hepcidin in order to increase iron absorption and recycling.2
Hepcidin is an iron-regulated hepatic peptide hormone that controls iron absorption at the intestinal level, and iron release from macrophages and hepatocytes.3,4 Hepcidin binds to the plasma membrane iron exporter ferroportin and induces its endocytosis and degradation, preventing release of iron into the plasma.5 Hepcidin expression is regulated through the bone morphogenetic protein–mothers against decapentaplegic homolog (BMP-SMAD) pathway.6 BMP6,7,8 a member of the transforming growth factor-β superfamily, binds to a complex of BMP receptors and the co-receptor hemojuvelin (HJV),9 leading to the phosphorylation of the SMAD1/5/8 proteins, which translocate to the nucleus after complexing with SMAD4.10
When iron requirements of erythroid progenitor cells are increased, hepcidin expression is repressed by one or several circulating erythroid factors produced by the bone marrow (BM),11 in order to increase dietary iron absorption and release iron stored in hepatocytes and macrophages. Although several candidates have been proposed, such as growth differentiation factor 15 (GDF15)12 and twisted gastrulation BMP signaling modulator 1 (TWSG1),13 it has been recently demonstrated that erythroferrone (ERFE)14 is the circulating erythroid factor responsible for hepcidin suppression in acute response to erythroid stress. ERFE is an EPO-responsive gene. Phlebotomized mice or mice injected with EPO rapidly induce Erfe messenger RNA (mRNA) expression in erythroid precursors in the BM and the spleen. In addition, Erfe knockout (KO) mice fail to suppress hepcidin acutely in response to phlebotomy or EPO injections, indicating that ERFE is required for rapid hepcidin suppression in the setting of increased erythroid activity.
Matriptase-2 (encoded by the TMPRSS6 gene) is a serine protease expressed mainly in the liver. In humans, mutations in the TMPRSS6 gene are responsible for a hereditary autosomal recessive disorder characterized by iron-refractory iron-deficiency anemia (IRIDA).15 Patients and matriptase-2–deficient mice display microcytic hypochromic anemia, low serum iron, and reduced transferrin saturation due to high hepcidin level.16,17 In vitro studies have shown that matriptase-2 cleaves HJV,18 attenuating the BMP-SMAD pathway activation and reducing hepcidin expression.
The relationship between ERFE and the in vivo hepatic inhibitor of hepcidin, matriptase-2, remains to be clarified. Indeed, despite anemia and high serum EPO levels, hepcidin is inappropriately high in IRIDA patients and in Tmprss6 KO mice. Attempts have been made to improve anemia and reduce hepcidin expression in IRIDA patients and Tmprss6 KO mice with injections of EPO. However, in the absence of matriptase-2, EPO injections do not correct anemia,19,20 suggesting that either hepcidin expression is too high to be significantly reduced by EPO, or matriptase-2 is required for hepcidin repression by erythroid stress. The role of matriptase-2 in erythropoiesis-mediated hepcidin regulation is further strengthened by studies focused on genetic loss or inactivation of Tmprss6 in the context of β-thalassemia. β-thalassemia is a recessive disorder characterized by decreased synthesis of β-globin chains, ineffective erythropoiesis, and secondary iron overload due to hepcidin downregulation. Tmprss6 germinal inactivation21 or Tmprss6 downregulation by short interfering RNA22 or antisense oligonucleotides23 in thalassemia Hbbth3/+ mice rescue iron overload and ameliorate anemia by increasing hepcidin levels and restricting the iron supply to erythropoiesis. In the Tmprss6-Hbbth3/+ double mutant animals, hepcidin expression remains high notwithstanding high EPO levels, suggesting that matriptase-2 is indispensable for hepcidin inhibition by the erythroid regulator(s).
To unequivocally elucidate the role of matriptase-2 in EPO-mediated hepcidin downregulation, we have treated Tmprss6 KO mice in comparison with iron-deficient (ID) and iron-replete littermates with a single EPO injection, and characterized the erythroid and hepcidin responses at a short time after injection.
We demonstrate that EPO efficiently inhibits hepcidin in ID mice, but not in Tmprss6 KO mice. Lack of hepcidin inhibition occurs also in wild-type (WT) animals when the Bmp-Smad signaling is upregulated by iron overload, suggesting that high BMP-SMAD signaling blunts ERFE-mediated hepcidin suppression. However, loss of Bmp6 in Tmprss6 KO mice, where the pathway signaling activity is similar to that of WT mice, does not rescue Erfe responsiveness. Interestingly, further pharmacologic inhibition of BMP type I receptors with dorsomorphin (DM) partially improves Erfe-mediated hepcidin suppression, suggesting that the activation level of the specific Tmprss6-Hjv-SMAD pathway influences the function of Erfe.
Materials and methods
Mice models, diets, and treatments
Animals were maintained in the animal facilities of INSERM US006 and the San Raffaele Scientific Institute (Milan, Italy) in accordance with European Union guidelines. The studies were approved by the Midi-Pyrénées Animal Ethics Committee and the Institutional Animal Care and Use Committee of the San Raffaele Scientific Institute. Animals were given free access to tap water and standard laboratory mouse chow diet (250 mg iron/kg; SAFE, Augy, France).
C57BL/6-Tmprss6 KO mice
Tmprss6 KO mice on a mixed genetic background (129/OlaxC57BL/6) were bred by 10 successive backcrosses onto the C57BL/6 background. Males were analyzed at 8 to 11 weeks of age and compared with C57BL/6 WT mice. To generate double Bmp6-Tmprss6 KO mice, C57BL/6 Tmprss6 KO animals were bred with CD1 Bmp6 KO mice and heterozygous F1 mice were then intercrossed in order to obtain F2 littermates. Experiments were performed on 9-week-old males.
Mixed 129/OlaC57BL/6 background-Tmprss6 KO mice
Tmprss6 KO mice (males and females, 4- to 5-weeks old)17 and control littermates were maintained an iron balanced (IB; carbonyle iron 200 mg/kg; SAFE) or an ID diet with virtually no iron (<3 mg iron/kg; SAFE) for 3 weeks to induce stable changes of the iron status. To inactivate the BMP-SMAD pathway, WT and Tmprss6 KO mice were treated with 2 doses of DM (12.5 μg/g in dimethylsulfoxide; intraperitoneal [IP] injection) (Sigma-Alrich) every 8 hours. The second DM injection was combined with EPO (200 U; see section to follow) or saline administration. Mice were euthanized 15 hours after the EPO/saline injection.
For EPO treatment, mice from a pure or mixed genetic background received a single IP dose of human EPO (200 U) (EMD Millipore, Billerica, MA or Eprex, Janssen) and were analyzed 15 hours later.
To induce severe iron overload, sv129 WT mice were treated with a single IP injection of iron dextran (1 g/kg; Sigma-Aldrich) and analyzed 1 week later. To induce mild iron overload, CD1 female (6-weeks old) received one subcutaneous injection of iron dextran weekly for 2 weeks (185 mg/kg).
Animals were anesthetized and euthanized by cervical dislocation. All efforts were made to minimize suffering. Livers, spleens, and kidneys were snap-frozen for isolation of RNA or fixed in 4% buffered formalin and embedded in paraffin. Livers and spleen were dried for tissue iron content analysis. Spleens were processed for erythropoiesis analysis. BM cells were isolated by flushing of femurs and snap-frozen for RNA isolation or processed for erythropoiesis analysis (see section to follow).
Analysis of hematologic and iron parameters
When euthanized, blood was collected for serum preparation and for complete blood count analysis with a CELL-DYN Emerald system (Abbott, Lake Forest, IL) or using a Sysmex KX-21 automated blood cell analyzer (Sysmex America).
Iron parameters such as transferrin saturation, serum iron, liver iron content (LIC), and spleen iron content (SIC) were analyzed as previously described24,25 and according to the method recommended by Torrance.26 Briefly, 100 mg of tissue were homogenized with 150 μL water in a FastPrep-24 Instrument (MP Biomedicals, Santa Ana, CA) for 60 seconds at 6 m/s, mixed with a solution of HCl 30%/trichloracetic acid 10% in a final volume of 1.5 mL, and kept overnight at 65°C before performing the colorimetric assay.
Deparaffinized tissue sections were stained with the Perls’ Prussian blue stain for non-heme iron and counterstained with nuclear fast red. Slides were scanned on a Pannoramic 250 Flash II (3DHISTECH) and analyzed with the Pannoramic Viewer software.
Cell preparations and flow cytometry
Cell suspensions from BM and the spleen were filtered through a 70-µm cell strainer and centrifuged at 350 g for 10 minutes. Cell pellets were suspended in phosphate-buffered saline containing 0.1% bovine serum albumin. To analyze erythroid precursors, cells were incubated with phycoerythrin-Cy7–conjugated anti-CD11b (clone M1/70), phycoerythrin-Cy7–conjugated anti-B220 (clone RA3-6B2), fluorescein isothiocyanate-conjugated anti–TER-119 (clone TER-119), and antigen presenting cell-conjugated anti-CD44 (clone IM7) as described.27 The listed antibodies were from BD Biosciences. After washing, cells were analyzed on the Navios Flow Cytometer (Beckman Coulter). Analyses were performed with the FCS Express software (De Novo Software).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Detailed experimental procedures are described in supplemental Materials and Methods available on the Blood Web site. Briefly, total RNA was isolated using Isol-RNA lysis reagent or Trizol reagent. RNAs from BM cells were isolated by flushing of femurs with 1 mL of Trizol or the RNeasy Mini Kit. First-strand complementary DNA synthesis was performed using Moloney murine leukemia virus–reverse transcriptase or using the High Capacity cDNA Reverse Transcription Kit. Gene expression levels were measured by real-time quantitative PCR using TaqMan Gene Expression Master Mix, SYBR Green Gene Expression Master Mix, or LightCycler 480 DNA SYBR Green I Master Reaction Mix. Results are expressed as mean ± standard deviation (SD). Values shown are means of −ΔCt (ie, Ct Hprt − Ct target). The higher the −ΔCt, the greater is the amount of target amplicon. Estimates of the fold changes in gene expression (2−ΔΔCt) are shown on the graphs.
Western blot analysis
Detailed experimental procedures are described in supplemental Materials and Methods. Briefly, livers were homogenized in lysis buffer and equal amounts of proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes. Immunoblots were performed with p-Smad5, Smad5, and vinculin antibodies.
Enzyme-linked immunosorbent assay
Serum hepcidin levels were quantified using the Intrinsic LifeSciences (La Jolla, CA) Hepcidin-Murine Compete competitive enzyme-linked immunosorbent assay. Serum EPO levels were measured using the mouse EPO Quantikine set (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Statistical analysis
Data are presented as mean ± SD or standard error of the mean (SEM) as indicated in the figure legends. Means of liver and SIC, analysis of erythroid precursors, quantitative PCR ΔCt values, serum hepcidin, and serum Epo in mice were compared with Student t tests or with one-way analysis of variance (ANOVA), followed by Sidak’s or by Newman–Keuls multiple comparison tests to test planned contrasts between pairs of means as indicating in the figure legends.
Results
EPO does not suppress hepcidin in Tmprss6 KO mice
As already published, Tmprss6 KO mice are anemic compared with C57BL/6 WT animals (Table 1), have lower LIC (Figure 1A), and are associated with increased hepcidin mRNA expression (Figure 1B) and higher serum hepcidin levels (Figure 1C). In addition, Tmprss6 KO mice have increased circulating Epo levels (Figure 1D) and induction of Erfe mRNA expression in the BM (Figure 1E), indicating that sensing of anemia is retained when matriptase-2 is lost.
Hematologic parameters of C57BL/6 WT and Tmprss6 KO mice on a C57BL/6 pure genetic background
| . | RBC × 106 cells/μL . | Hb (g/dL) . | HCT (%) . | MCV (fL) . | MCH (pg) . | RDW (%) . |
|---|---|---|---|---|---|---|
| B6 WT | 9.4 ± 0.8 | 15 ± 1.2 | 41 ± 3 | 43.9 ± 1 | 16 ± 0.3 | 36.6 ± 0.4 |
| Tmprss6−/− | 9.1 ± 1.3 | 11.6 ± 1.2* | 27.6 ± 4.2† | 30.3 ± 0.5† | 12.9 ± 0.6† | 42.4 ± 1.7† |
| . | RBC × 106 cells/μL . | Hb (g/dL) . | HCT (%) . | MCV (fL) . | MCH (pg) . | RDW (%) . |
|---|---|---|---|---|---|---|
| B6 WT | 9.4 ± 0.8 | 15 ± 1.2 | 41 ± 3 | 43.9 ± 1 | 16 ± 0.3 | 36.6 ± 0.4 |
| Tmprss6−/− | 9.1 ± 1.3 | 11.6 ± 1.2* | 27.6 ± 4.2† | 30.3 ± 0.5† | 12.9 ± 0.6† | 42.4 ± 1.7† |
Complete blood counts were measured from whole blood. Data are presented as mean ± SD. Group sizes: B6 WT (n = 5) and Tmprss6 KO (n = 8).
HCT, hematocrit; MCH, mean corpuscular Hb; MCV, mean corpuscular volume; RDW, red cell distribution width.
P < .05. Tmprss6 KO mice vs B6 WT mice.
P < .0001.
Tmprss6−/− mice sense anemia but do not respond to EPO. C57BL/6 WT or Tmprss6 KO mice were non-injected or injected with EPO (5 to 8 mice per group) and were addressed 15 hours later for (A) liver non-heme iron content; (B) liver Hamp mRNA expression; (C) serum hepcidin levels; (D) serum Epo levels; (E) BM Erfe mRNA expression; and (F) liver pSmad-5 relative to total Smad5 protein. Results are expressed as mean ± SD and compared by Student t tests (A,D) or by ANOVA followed by Sidak’s multiple comparison tests. ****P < .0001; ***P < .001; **P < .01; *P < .05. Point estimates of the fold changes in gene expression (2−ΔΔCt) are shown on the graphs.
Tmprss6−/− mice sense anemia but do not respond to EPO. C57BL/6 WT or Tmprss6 KO mice were non-injected or injected with EPO (5 to 8 mice per group) and were addressed 15 hours later for (A) liver non-heme iron content; (B) liver Hamp mRNA expression; (C) serum hepcidin levels; (D) serum Epo levels; (E) BM Erfe mRNA expression; and (F) liver pSmad-5 relative to total Smad5 protein. Results are expressed as mean ± SD and compared by Student t tests (A,D) or by ANOVA followed by Sidak’s multiple comparison tests. ****P < .0001; ***P < .001; **P < .01; *P < .05. Point estimates of the fold changes in gene expression (2−ΔΔCt) are shown on the graphs.
In contrast to the substantial decrease of hepcidin expression observed in WT mice injected with EPO, hepcidin was not significantly reduced by EPO in Tmprss6 KO mice (Figure 1B) despite a further increase in Erfe expression in the BM of these mice (Figure 1E). As shown in Figure 1B-C, serum hepcidin levels reflect liver mRNA expression. EPO injection neither reduces serum hepcidin (Figure 1C) nor affects LIC (Figure 1A). Altogether, these results suggest that either loss of matriptase-2 or persistent iron deficiency prevents the inhibition of hepcidin expression by Erfe.
EPO-induced erythropoiesis changes and Erfe expression
To investigate the contribution of iron deficiency or lack of matriptase-2 in hepcidin responsiveness to EPO, the phenotype of Tmprss6 KO animals on a mixed background was compared with WT and heterozygous littermates maintained for 3 weeks with an IB or ID diet. As expected, ID WT mice were anemic compared with IB animals (data not shown). However, the degree of iron deficiency anemia in Tmprss6 KO mice was more severe than in ID animals (Figure 2A-B), although Epo serum levels (Figure 2C) were comparable between the two groups of mice.
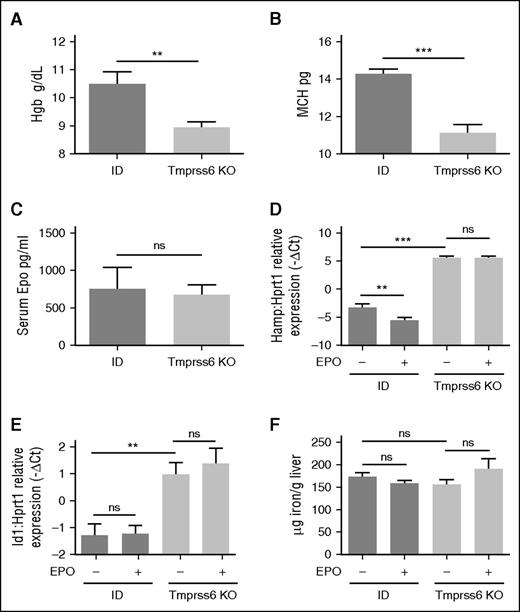
Hematologic parameters and iron homeostasis in EPO-treated ID and Tmprss6 KO mice. WT littermates were fed an ID diet for 3 weeks and analyzed in comparison with Tmprss6 KO animals on a mixed genetic background. Hb levels (A) and MCH (B) are shown from 4 to 10 mice per group. Serum EPO (C) was measured in ID mice (n = 5) and Tmprss6 KO animals (n = 6). The BMP-SMAD target genes Hamp (D) and Id1 (E) were analyzed by qRT-PCR in liver samples from Tmprss6 KO animals, on a mixed background, and control littermates (6 to 13 mice per group) kept an ID diet for 3 weeks and were injected with saline or EPO. mRNA expression was normalized to the housekeeping gene Hprt1. (F) Non-heme LIC was measured in saline- or EPO-treated ID and Tmprss6 KO mice. Error bars indicate ± SEM. **P < .01; ***P < .001. ns, nonsignificant.
Hematologic parameters and iron homeostasis in EPO-treated ID and Tmprss6 KO mice. WT littermates were fed an ID diet for 3 weeks and analyzed in comparison with Tmprss6 KO animals on a mixed genetic background. Hb levels (A) and MCH (B) are shown from 4 to 10 mice per group. Serum EPO (C) was measured in ID mice (n = 5) and Tmprss6 KO animals (n = 6). The BMP-SMAD target genes Hamp (D) and Id1 (E) were analyzed by qRT-PCR in liver samples from Tmprss6 KO animals, on a mixed background, and control littermates (6 to 13 mice per group) kept an ID diet for 3 weeks and were injected with saline or EPO. mRNA expression was normalized to the housekeeping gene Hprt1. (F) Non-heme LIC was measured in saline- or EPO-treated ID and Tmprss6 KO mice. Error bars indicate ± SEM. **P < .01; ***P < .001. ns, nonsignificant.
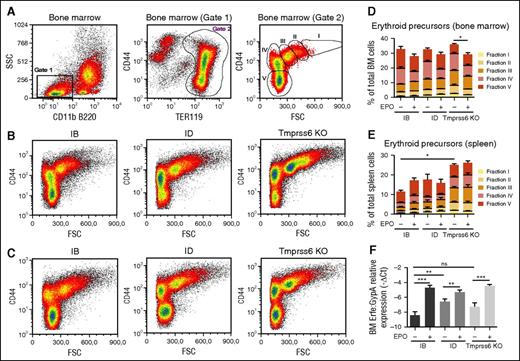
We analyzed BM and spleen erythroid precursors in basal conditions and after EPO injection in WT and Tmprss6 KO mice. To identify maturing erythroblasts in BM and the spleen, we considered side scatter low CD11b− B220− TER119+ cells, plotting CD44 expression vs forward scatter value, as previously described.28 We identified 5 density clusters corresponding to erythroblast populations of progressive maturation stages (I-V) (Figure 3A and supplemental Figure 1A).
Flow cytometry analysis of BM erythropoiesis and ERFE expression. (A) Identification of clusters of BM erythroid precursors in WT littermates. (Left) Identification of SSC low CD11b− and B220− cells (gate 1). (Middle) Recognition of erythroid precursors (TER119+ cells, gate 2) inside the population identified in gate 1. (Right) Density plot of CD44 vs FSC of cells identified in gate 2 showing naturally occurring clusters of erythroid precursors at progressive maturation stages (fractions I-V). (B) Density plots of TER119+ cells in WT littermates fed the IB or ID diet and in Tmprss6 KO mice on a mixed genetic background showing representative distribution of erythroid precursors. (C) Density plots showing representative distribution of TER119+ cells after treatment with EPO for 15 hours. (D) Quantification of BM erythroid fractions I-V with respect of total BM cells. (E) Quantification of spleen erythroid fractions I-V identified as in (A) on total spleen cells. Quantification was performed on samples from at least 6 mice for every condition. Each erythroid fraction is calculated as a percentage of total BM or spleen cells. Quantitative variations of each erythroid fraction among different mice groups are listed in supplemental Tables 1-4. (F) Tmprss6 KO animals, on a mixed genetic background, were analyzed in comparison with control littermates fed an IB or an ID diet for 3 weeks. Erfe expression was analyzed by qRT-PCR in BM derived cells from 6 to 3 mice per group. mRNA expression was normalized relative to the erythroid marker GypA. Error bars indicate ± SEM. *P < .05; **P < .01; ***P < .001. FSC, forward side scatter; ns, nonsignificant; SSC, side scatter.
Flow cytometry analysis of BM erythropoiesis and ERFE expression. (A) Identification of clusters of BM erythroid precursors in WT littermates. (Left) Identification of SSC low CD11b− and B220− cells (gate 1). (Middle) Recognition of erythroid precursors (TER119+ cells, gate 2) inside the population identified in gate 1. (Right) Density plot of CD44 vs FSC of cells identified in gate 2 showing naturally occurring clusters of erythroid precursors at progressive maturation stages (fractions I-V). (B) Density plots of TER119+ cells in WT littermates fed the IB or ID diet and in Tmprss6 KO mice on a mixed genetic background showing representative distribution of erythroid precursors. (C) Density plots showing representative distribution of TER119+ cells after treatment with EPO for 15 hours. (D) Quantification of BM erythroid fractions I-V with respect of total BM cells. (E) Quantification of spleen erythroid fractions I-V identified as in (A) on total spleen cells. Quantification was performed on samples from at least 6 mice for every condition. Each erythroid fraction is calculated as a percentage of total BM or spleen cells. Quantitative variations of each erythroid fraction among different mice groups are listed in supplemental Tables 1-4. (F) Tmprss6 KO animals, on a mixed genetic background, were analyzed in comparison with control littermates fed an IB or an ID diet for 3 weeks. Erfe expression was analyzed by qRT-PCR in BM derived cells from 6 to 3 mice per group. mRNA expression was normalized relative to the erythroid marker GypA. Error bars indicate ± SEM. *P < .05; **P < .01; ***P < .001. FSC, forward side scatter; ns, nonsignificant; SSC, side scatter.
Iron deficiency induced both by an ID diet and by the lack of Tmprss6 affects BM erythroid precursors, causing a significant reduction of the final step (stage V) of maturation and leads to the increase of immature populations (stages I-III), a finding more evident in Tmprss6 KO mice (Figure 3B,D and supplemental Table 1). In all mice, EPO treatment reduces BM stage IV favoring maturation into stage V (Figure 3B,D and supplemental Table 3). Iron deficiency increases erythroid precursors (stages I-V) in the spleen mainly in Tmprss6 KO mice (Figure 3E, supplemental Figure 1A-B, and supplemental Table 2). EPO enhances splenic erythropoiesis in iron-replete mice, whereas it does not further increase erythroid precursors in ID and in Tmprss6 KO animals (Figure 3E, supplemental Figure 1B-C, and supplemental Table 4), likely because stress erythropoiesis is already present in these conditions. From these results, we conclude that EPO stimulates BM erythropoiesis both in IB and ID WT animals and in Tmprss6 KO mice.
Because EPO modulates erythropoiesis, we investigated Erfe expression in these animals. Erfe is highly expressed in the BM of ID and Tmprss6 KO mice consistent with their high Epo levels (Figure 2C). The upregulation of BM Erfe is due to increased expression of Erfe in erythroid cells (Figure 3F). EPO injection further increases Erfe expression both in WT and Tmprss6 KO animals, suggesting that the response to EPO is maintained in conditions of acute (ID) or chronic (Tmprss6 KO) iron deficiency. To investigate whether iron deficiency modulates the EPO-dependent response, we analyzed liver hepcidin expression in ID compared with Tmprss6 KO mice. In untreated animals, hepcidin is strongly reduced in ID mice, whereas it is inappropriately high in Tmprss6 KO mice as expected. EPO injection inhibits hepcidin in ID animals but not in Tmprss6 KO animals (Figure 2D) despite erythropoiesis expansion and a further increase of Erfe mRNA expression (Figure 2F). Id1 expression (Figure 2B) and LIC (Figure 2C) were unchanged after EPO treatment. These results indicate that inactivation of matriptase-2 impairs EPO-mediated hepcidin inhibition.
Activated BMP-SMAD pathway blunts hepcidin repression by EPO
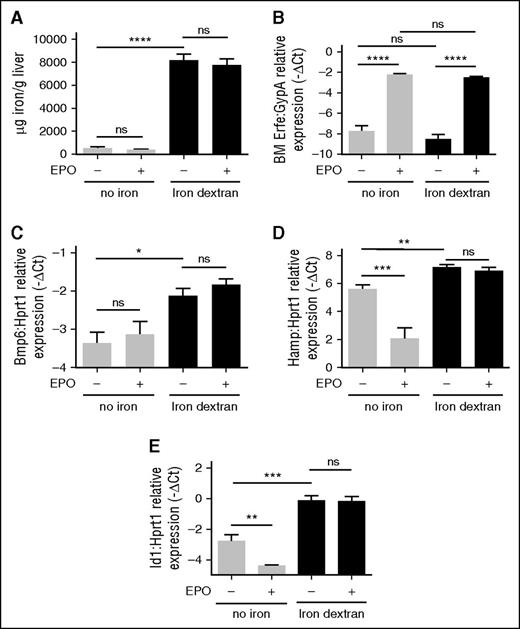
It has been reported that hepcidin suppression by EPO/Erfe occurs independently from the BMP-SMAD signaling, because Hjv KO mice, who have the pathway downregulated,9 efficiently inhibit hepcidin after phlebotomy.14,29 Our results show that in WT mice EPO treatment decreases Smad5 phosphorylation (Figure 1F and supplemental Figure 2A-B), suggesting that the BMP-SMAD pathway may contribute to the Erfe-mediated hepcidin suppression. Interestingly, Smad5-phosphorylation is not reduced by EPO in Tmprss6 KO mice, in which BMP signaling is activated (Figure 1F and supplemental Figure 2A-B). Altogether, these results suggest that hyperactivation of the BMP-SMAD pathway interferes with Erfe function. To test this hypothesis, we treated WT mice with a single IP injection of iron dextran (1g/kg) to activate the pathway and then challenged the mice with a EPO injection 1 week later. Iron-dextran–treated mice show a strong increase in LIC (Figure 4A) and upregulation of the BMP-SMAD signaling pathway, because Bmp6 (Figure 4C), Hamp (Figure 4D), and Id1 (Figure 4E) are all increased. EPO strongly upregulates BM Erfe expression in both iron-replete and iron-loaded animals (Figure 4B). However, in iron-loaded animals, hepcidin (Figure 4D) and Id1 (Figure 4E) are not reduced by EPO, similar to the pattern observed during inactivation of matriptase-2. The lack of Erfe responsiveness is not due to the degree of iron overload, because animals treated with a protocol that induces a milder iron overload also do not downregulate hepcidin when challenged with EPO (supplemental Figure 3B) and in response to increased Erfe expression (supplemental Figure 3C). Overall, these data suggest that hyperactivation of the BMP-SMAD pathway interferes with the Erfe function.
Modulation of Erfe, hepcidin, and Id1 by EPO in iron-loaded mice. Sv129 WT mice were injected with iron dextran and challenged with EPO or vehicle 1 week later. Control mice (no iron) were treated with saline. Non-heme LIC (A) was measured in untreated and iron-loaded mice. Erfe expression was measured by qRT-PCR in BM-derived cells. mRNA expression was normalized relative to the erythroid marker GypA (B). Four mice per group were analyzed. Bmp6 mRNA expression (C), BMP-SMAD target genes as Hamp (D), and Id1 (E) were analyzed by qRT-PCR in total liver of treated animals and normalized to the housekeeping gene Hprt1. Error bars indicate ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001. ns, nonsignificant.
Modulation of Erfe, hepcidin, and Id1 by EPO in iron-loaded mice. Sv129 WT mice were injected with iron dextran and challenged with EPO or vehicle 1 week later. Control mice (no iron) were treated with saline. Non-heme LIC (A) was measured in untreated and iron-loaded mice. Erfe expression was measured by qRT-PCR in BM-derived cells. mRNA expression was normalized relative to the erythroid marker GypA (B). Four mice per group were analyzed. Bmp6 mRNA expression (C), BMP-SMAD target genes as Hamp (D), and Id1 (E) were analyzed by qRT-PCR in total liver of treated animals and normalized to the housekeeping gene Hprt1. Error bars indicate ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001. ns, nonsignificant.
Inhibition of the Bmp6-dependent Smad pathway does not restore hepcidin regulation by EPO in Tmprss6 KO mice
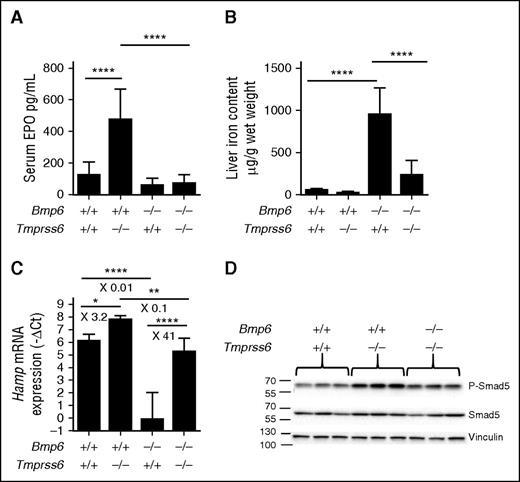
To confirm that high levels of the BMP-SMAD signaling impair the EPO-mediated suppression of hepcidin in Tmprss6 KO mice, we genetically inactivated Bmp6, one of the main activators of the pathway, in the Tmprss6 KO animals. In Figure 5, we compare WT, single Tmprss6 or Bmp6 KO, and double KO littermates. As expected, Tmprss6 KO mice are anemic (Table 2), have high Epo levels (Figure 5A), and increased hepcidin expression (Figure 5C), whereas Bmp6 KO mice have increased LIC (Figure 5B), iron accumulation in extrahepatic tissues such as the pancreas (supplemental Figure 4), and reduced hepcidin levels (Figure 5C).
Bmp6−/−-Tmprss6−/− mice are neither anemic nor iron overloaded. F2 littermate mice (CD1-C57BL/6 mixed background) of the different genotypes (5 to 9 mice per group) were analyzed for (A) serum EPO level; (B) liver non-heme iron content; (C) liver Hamp mRNA expression; and (D) liver pSmad-5 relative to total Smad5 protein. Results are expressed as mean ± SD and compared by ANOVA followed by Sidak’s multiple comparison tests. ****P < .0001; **P < .01; *P < .05. Point estimates of the fold changes in gene expression (2-ΔΔCt) are shown on the graphs.
Bmp6−/−-Tmprss6−/− mice are neither anemic nor iron overloaded. F2 littermate mice (CD1-C57BL/6 mixed background) of the different genotypes (5 to 9 mice per group) were analyzed for (A) serum EPO level; (B) liver non-heme iron content; (C) liver Hamp mRNA expression; and (D) liver pSmad-5 relative to total Smad5 protein. Results are expressed as mean ± SD and compared by ANOVA followed by Sidak’s multiple comparison tests. ****P < .0001; **P < .01; *P < .05. Point estimates of the fold changes in gene expression (2-ΔΔCt) are shown on the graphs.
Hematologic parameters of Bmp6-Tmprss6 genotype combinations
| . | RBC × 106 cells/μL . | Hb (g/dL) . | HCT (%) . | MCV (fL) . | MCH (pg) . | RDW (%) . |
|---|---|---|---|---|---|---|
| Bmp6+/+-Tmprss6+/+ | 10.4 ± 0.9 | 17.1 ± 1.4 | 46.5 ± 4.4 | 44.6 ± 1.8 | 16.4 ± 0.7 | 27.4 ± 1.4 |
| Bmp6+/+-Tmprss6−/− | 9.5 ± 0.6 | 12.1 ± 0.8* | 30.9 ± 2.7* | 32.4 ± 1.3* | 12.7 ± 0.3* | 35.5 ± 1.4* |
| Bmp6−/−-Tmprss6+/+ | 9.5 ± 0.8 | 16.7 ± 0.8 | 44.1 ± 2.8 | 46.7 ± 1.8 | 17.8 ± 1.2 | 25.1 ± 5 |
| Bmp6−/−-Tmprss6−/− | 9.8 ± 0.8 | 16.4 ± 1.3† | 44.2 ± 3.6† | 45 ± 1.3† | 16.7 ± 0.5† | 27.5 ± 2† |
| . | RBC × 106 cells/μL . | Hb (g/dL) . | HCT (%) . | MCV (fL) . | MCH (pg) . | RDW (%) . |
|---|---|---|---|---|---|---|
| Bmp6+/+-Tmprss6+/+ | 10.4 ± 0.9 | 17.1 ± 1.4 | 46.5 ± 4.4 | 44.6 ± 1.8 | 16.4 ± 0.7 | 27.4 ± 1.4 |
| Bmp6+/+-Tmprss6−/− | 9.5 ± 0.6 | 12.1 ± 0.8* | 30.9 ± 2.7* | 32.4 ± 1.3* | 12.7 ± 0.3* | 35.5 ± 1.4* |
| Bmp6−/−-Tmprss6+/+ | 9.5 ± 0.8 | 16.7 ± 0.8 | 44.1 ± 2.8 | 46.7 ± 1.8 | 17.8 ± 1.2 | 25.1 ± 5 |
| Bmp6−/−-Tmprss6−/− | 9.8 ± 0.8 | 16.4 ± 1.3† | 44.2 ± 3.6† | 45 ± 1.3† | 16.7 ± 0.5† | 27.5 ± 2† |
Complete blood counts were measured from whole blood. Data are presented as mean ± SD. Group sizes: Bmp6+/+-Tmprss6+/+ (n = 6); Bmp6+/+-Tmprss6−/− (n = 6); Bmp6−/−-Tmprss6+/+ (n = 7); and Bmp6−/−-Tmprss6−/− (n = 5). Bmp6−/−-Tmprss6−/− mice vs Bmp6+/+-Tmprss6+/+ mice; no statistical significance was observed.
P < .0001. Bmp6+/+-Tmprss6−/− mice vs Bmp6+/+-Tmprss6+/+ mice.
P < .0001. Bmp6−/−-Tmprss6−/− mice vs Bmp6+/+-Tmprss6−/− mice.
Interestingly, the deletion of Bmp6 in Tmprss6 KO mice alleviates anemia (Table 2) and normalizes Epo levels (Figure 5A). In contrast to Tmprss6 KO, hepcidin expression (Figure 5C) is lower in the double KO mice, reaching levels comparable to WT animals. As a consequence, Bmp6-Tmprss6 double KO mice are not as iron loaded as Bmp6 KO animals (Figure 5B and supplemental Figure 4). Indeed, their liver iron accumulation is mild (or absent for some of them) and is essentially periportal instead of centrolobular as observed in Bmp6 KO mice (supplemental Figure 4). Furthermore, iron is not accumulated in extrahepatic tissues such as the pancreas, kidney, and heart (supplemental Figure 4). Consistent with these findings, Smad5 phosphorylation appears reduced in the double KO animals (Figure 5D and supplemental Figure 2C-D) compared with Tmprss6 KO animals, confirming that inactivation of Bmp6 mitigates the Bmp-Smad signaling in Tmprss6 KO mice. This makes the double KO model an interesting tool to examine the relationship between Erfe and hepcidin regulation in the absence of matriptase-2.
We compared the response of WT, Tmprss6 KO, Bmp6 KO, and Bmp6-Tmprss6 double KO littermates to EPO injection. All mice upregulate BM Erfe by EPO, independently of the genotype (Figure 6A). However, whereas EPO efficiently inhibits hepcidin in WT and Bmp6 KO mice (Figure 6B), in the Bmp6-Tmprss6 double KO animals it does not, despite the absence of anemia and a degree of activation of the Bmp pathway within the range observed in WT mice.
Despite normal Bmp-Smad signaling in Bmp6−/−-Tmprss6−/− mice, hepcidin is not repressed in response to EPO. F2 littermates mice (CD1-C57BL/6 mixed background) of the different genotypes (5 to 9 mice per group) were injected or not with EPO and analyzed 15 hours later for (A) BM Erfe mRNA expression; and (B) liver Hamp mRNA expression. Results are expressed as mean ± SD and compared by ANOVA followed by Sidak’s multiple comparison tests. ****P < .0001; ***P < .001; **P < .01. Point estimates of the fold changes in gene expression (2-ΔΔCt) are shown on the graphs. NS, non-significant.
Despite normal Bmp-Smad signaling in Bmp6−/−-Tmprss6−/− mice, hepcidin is not repressed in response to EPO. F2 littermates mice (CD1-C57BL/6 mixed background) of the different genotypes (5 to 9 mice per group) were injected or not with EPO and analyzed 15 hours later for (A) BM Erfe mRNA expression; and (B) liver Hamp mRNA expression. Results are expressed as mean ± SD and compared by ANOVA followed by Sidak’s multiple comparison tests. ****P < .0001; ***P < .001; **P < .01. Point estimates of the fold changes in gene expression (2-ΔΔCt) are shown on the graphs. NS, non-significant.
Overall, these results indicate that normalization of the Bmp-Smad signaling pathway through the deletion of Bmp6 is not sufficient to rescue EPO responsiveness in the absence of matriptase-2.
Downregulation of Bmp type I receptors by DM partially improves the hepcidin inhibition by EPO in Tmprss6 KO mice
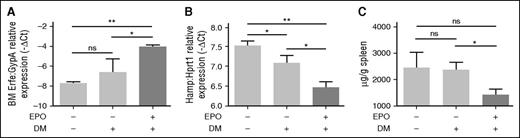
The evidence that genetic loss of Tmprss6 in Bmp6 KO animals activates hepcidin as in WT mice led us to hypothesize that Bmp6 and Tmprss6 are working on two different pathways, as recently proposed.30 To inhibit the Bmp type I receptors downstream of Bmp6, Tmprss6 KO animals on a mixed background were treated with DM.31 First, we show that DM does not interfere with BM Erfe upregulation in WT animals (supplemental Figure 5A), and that EPO treatment does not interfere with the DM inhibitory effect on hepcidin (supplemental Figure 5B) and Id1 (supplemental Figure 5C). Then, Tmprss6 KO mice were injected with DM or vehicle in the presence of EPO. DM after EPO induces Erfe upregulation in BM (Figure 7A), decreasing both hepcidin (Figure 7B) and Id1 (supplemental Figure 6A) in Tmprss6 KO mice, without changing the liver iron concentration (supplemental Figure 6B). The reduction of hepcidin is sufficient to decrease the SIC (Figure 7C). Overall, these data demonstrate that Bmp-Smad pathway inhibition downstream Bmp6 ameliorates hepcidin responsiveness to Erfe in the absence of matriptase-2.
Modulation of eErfe, hepcidin, and Id1 by EPO in DM-treated Tmprss6 KO mice.Tmprss6 KO mice (on a mixed background) were treated with vehicle (dimethylsulfoxide), DM, and DM plus EPO. Erfe expression was analyzed by qRT-PCR in (A) BM-derived cells and mRNA expression was normalized using the erythroid marker GypA. Liver expression of the BMP-SMAD target genes Hamp (B) was measured by qRT-PCR and normalized to the housekeeping gene Hprt1. Non-heme SIC (C) was measured. A total of 3 to 4 mice per group were analyzed. Error bars indicate ± SEM. *P < .05; **P < .01. ns, nonsignificant.
Modulation of eErfe, hepcidin, and Id1 by EPO in DM-treated Tmprss6 KO mice.Tmprss6 KO mice (on a mixed background) were treated with vehicle (dimethylsulfoxide), DM, and DM plus EPO. Erfe expression was analyzed by qRT-PCR in (A) BM-derived cells and mRNA expression was normalized using the erythroid marker GypA. Liver expression of the BMP-SMAD target genes Hamp (B) was measured by qRT-PCR and normalized to the housekeeping gene Hprt1. Non-heme SIC (C) was measured. A total of 3 to 4 mice per group were analyzed. Error bars indicate ± SEM. *P < .05; **P < .01. ns, nonsignificant.
Discussion
The optimal access of erythron to iron is ensured by an essential crosstalk between hepcidin expression and the iron needs for erythropoiesis. Hepcidin production is suppressed after hemorrhage, hemolysis, and other conditions that trigger EPO increase and stress erythropoiesis, so that absorption of dietary iron and release of iron from stores are increased.32 Several secreted erythroid factors have been identified as hepcidin suppressors: GDF15,12 TWSG113 and, more recently, ERFE.14 In contrast to GDF15 and TWSG1, that do not appear to be physiological suppressors of hepcidin in vivo, ERFE seems essential for recovering from an erythropoietic stress.
Severely anemic IRIDA patients and Tmprss6 KO mice have inappropriately high hepcidin levels16,17 and administration of EPO does not resolve their anemia.19,20 In addition, it has been previously reported that Tmprss6 deletion in β-thalassemia Hbbth3/+ mice, characterized by high Epo, low hepcidin, and iron accumulation, prevents iron overload and partially corrects anemia through hepcidin upregulation,21 despite high Erfe expression.33 So far, little is known about the mechanisms by which ERFE regulates hepcidin expression in hepatocytes and whether this process requires the iron-regulatory machinery, including the serine protease TMPRSS6.
In this study, we show that high hepcidin levels in Tmprss6 KO mice are not due to abnormal sensing of anemia, as erythropoiesis and Erfe mRNA expression are appropriately induced in Tmprss6 KO mice. These observations suggest a role for matriptase-2 in preventing EPO-mediated hepcidin downregulation. To address this possibility, we induced Erfe expression in Tmprss6 KO mice by a single EPO injection and analyzed mice at a time point (15 hours) where Erfe is increased and hepcidin fully suppressed, according to previously published results.14 Although EPO treatment in established iron deficiency is often ineffective34 unless accompanied by iron supplementation, we observed that early EPO responsiveness is preserved in acute (ID animals) and chronic (Tmprss6 KO mice) iron deficiency anemia. Indeed, in all mice, EPO affects maturation of erythroid precursors, inducing Erfe expression. Although Erfe properly suppresses hepcidin in EPO-treated ID animals, the genetic loss of Tmprss6 impairs EPO-dependent hepcidin inhibition, suggesting that Erfe response is inefficient in this model, independent from the iron deficiency anemia.
Inactivation of Tmprss6 inappropriately upregulates the Bmp-Smad signaling, leading to increased Smad5 phosphorylation and higher hepcidin levels compared with WT mice. Thus, erythroid factors such as Erfe, normally intended to remedy stress erythropoiesis, might not be efficient enough to counteract this high level of signaling. Indeed, the inhibitory effect of Erfe is lost when the Bmp-Smad pathway is hyperactive as shown in the iron-loaded animals. Consistent with our results, hepcidin can be induced by iron supplementation even in the presence of high Epo levels, such as in mice constitutively overexpressing Epo.35
To assess the role of matriptase-2 in EPO-mediated hepcidin suppression under the condition of reduced activation of the Bmp-Smad signaling, we genetically inactivated Bmp6 in Tmprss6 KO mice. To achieve this aim, the double Tmprss6-Bmp6 KO mice constitutes the ideal model, since the anemia is completely rescued and the activation of the Bmp-Smad pathway and hepcidin levels are comparable to those of WT mice. Although hepcidin is strongly decreased in Bmp6 KO mice, hepcidin mRNA expression in double Tmprss6-Bmp6 KO mice is not as low as in Bmp6 KO mice, as previously observed.36 Such a difference could be explained by the influence of the genetic background of the mice being studied, because our Tmprss6 KO mice are in a C57BL/6 pure genetic background, whereas the one from Lenoir et al are in mixed 129/Ola×C57BL/6 background. Interestingly, although Erfe mRNA expression in the BM of the double KO mice is induced by EPO, these mice are unable to suppress hepcidin when compared with WT animals with comparable activation of Bmp-Smad signaling, suggesting that Bmp6 and matriptase-2 control hepcidin through two independent pathways, in agreement with the recently proposed model.30
At variance with what we observed in Bmp6 KO animals, Tmprss6 inactivation in Hjv KO mice does not activate hepcidin, implying that Hjv and matriptase-2 are in the same hepcidin regulatory pathway. Consistent with this hypothesis, the Hjv-Tmprss6 double KO mice suppress hepcidin in response to EPO treatment.37
To further inhibit the pathway downstream of Bmp6, Tmprss6 KO animals were treated with DM, a compound that inhibits the Bmp type I receptors. Pharmacologic inhibition of the Bmp pathway partially improves EPO responsiveness, reducing hepcidin levels in Tmprss6 KO animals.
Overall, these findings suggest that activation of the Hjv-Bmp signaling pathway in the setting of the loss of Tmprss6 impairs appropriate hepcidin downregulation by EPO, possibly via Erfe. These data also exclude the hypothesis that protease activity of matriptase-2 on Erfe or its receptor(s) is required for hepcidin regulation.
Hepcidin inhibition has the final goal of increasing iron supply for erythropoiesis. The erythroid hepcidin-suppressive signals should be inactivated when iron is high to avoid further iron acquisition. For an accurate tuning of hepcidin levels, the function of ERFE should be coordinated with that of the BMP-SMAD activating pathways. By cleaving HJV, TMPRSS6 could rapidly reduce the BMP-SMAD signaling, to allow hepcidin suppression by ERFE in response to erythropoietic iron needs. In line with this hypothesis, Smad phosphorylation and Bmp-Smad target gene expression are reduced by EPO in WT mice, but not in Tmprss6 KO and in Tmprss6-Bmp6 double KO mice (supplemental Figure 7). Careful examination of the kinetics of hepcidin expression in Erfe KO mice shows a mild but significant (twofold) suppression of hepcidin 12 hours after phlebotomy,14 although the suppression is much more evident and sustained in WT mice. This early reduction of hepcidin expression is Erfe-independent and may well reflect the primary setting of the Bmp pathway inactivation that is Tmprss6 dependent.
Altogether, these data provide new insights on the regulation of hepcidin expression in response to acute erythropoietic stress. They suggest that in response to EPO, two signaling pathways are induced in parallel: the Erfe pathway and the matriptase-2 pathway. As demonstrated by Kautz et al,14 Erfe is produced by the erythroid progenitors and binds to its still unidentified receptor at the hepatocyte cell surface. In parallel, we hypothesize that matriptase-2 is activated or stabilized in response to EPO, independently of Erfe, in order to inhibit the Bmp-Smad signaling pathway. It is still unclear how matriptase-2 is activated or stabilized. We speculate that following EPO injection, the rapid erythropoiesis expansion may cause a transient drop of holo-transferrin and that transient iron deficiency can stabilize TMPRSS6/matriptase-2 on the cell surface.38 Following matriptase-2 stabilization, the Bmp-Smad pathway is inhibited through the Hjv-Bmp complex allowing a much more effective hepcidin inhibition by Erfe. Our data demonstrate that lack of Bmp-Smad signaling pathway inhibition prevents hepcidin suppression by Erfe. For an accurate tuning of hepcidin, the function of Erfe should be coordinated with that of the main hepcidin-activating pathway.
In conclusion, our data demonstrate for the first time that matriptase-2 is essential for hepcidin repression by increased erythropoiesis and that lowering the BMP-SMAD signaling is a prerequisite for the EPO function via ERFE.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Carlos Lopez Otin (University of Oviedo, Oviedo, Spain) for kindly providing the original Tmprss6−/− mice on a mixed genetic background and to Silvia Galvan (San Raffaele Scientific Institute, Milan, Italy) for technical support. They also thank Florence Capilla (Experimental Histopathology Platform, Toulouse Purpan), and members of the INSERM US006 facility (Toulouse) for their technical assistance and help in the mouse breeding.
This study was funded in part by grants from the Telethon Onlus Foundation (GGP12025), Ricerca Finalizzata (RF-2010-2312048), Ministero Sanità and Ministero dell’Istruzione dell’Universita e della Ricerca Progetto di Rilevante Interesse Nazionale (MIUR-PRIN 2010-2011) (C.C.), and by the Telethon Onlus Foundation (GGP15064) (L.S.). D.M. was supported by the Cooley’s Anemia Foundation award and a grant from the French Foundation for Rare Diseases. H.Y.L. was supported in part by a grant from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (RO1 DK-071837). H.C. and M.-P.R. were supported in part by a grant from the French National Research Agency (ANR, programme Genopat, project ANR-09-GENO-016).
Authorship
Contribution: A.N. and A.R. performed experiments, analyzed, and discussed results; A.C. performed experiments and analyzed data; I.A., J.B., O.G., A.G., C.L., and C.B.-F. performed experiments; H.Y.L. initiated the matriptase-2 project with D.M.; C.C., H.C., and M.-P.R. discussed data and wrote the manuscript; L.S. and D.M. designed research, performed experiments, analyzed and discussed data, and wrote the manuscript; and all authors reviewed and approved the final manuscript.
Conflict-of-interest disclosure: H.Y.L. has ownership interest in a start-up company (Ferrumax Pharmaceuticals), which has licensed technology from the Massachusetts General Hospital based on his work. The remaining authors declare no competing financial interests.
Correspondence: Delphine Meynard, IRSD INSERM U1220, CHU Purpan, BP 3048, 31024 Toulouse Cedex 3, France; e-mail: delphine.meynard@inserm.fr; Laura Silvestri, IRCCS San Raffaele Scientific Institute and Vita Salute University, Via Olgettina, 58, 20132 Milan, Italy; e-mail: silvestri.laura@hsr.it; and Clara Camaschella, IRCCS San Raffaele Scientific Institute and Vita Salute University, Via Olgettina, 58, 20132 Milan, Italy; e-mail: camaschella.clara@hsr.it.
References
Author notes
A.N. and A.R. contributed equally to this study.
L.S. and D.M. contributed equally to this study.