In this issue of Blood, by delving into the role of Janus kinase (Jak) signaling during formation of the first adult-repopulating hematopoietic stem cells (HSCs), Mascarenhas et al make the discovery that if HSCs are harvested from their site of emergence, they will undergo sustained normal hematopoiesis in the adult environment despite the presence of the potent pathogenic JAKV617F mutation.1
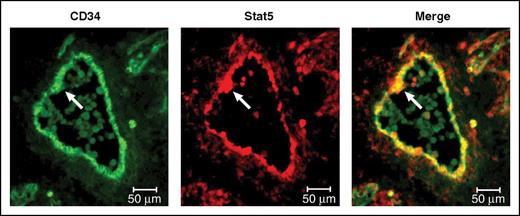
This panel adapted from Mascarenhas et al depicts the expression of total Stat5, which is activated by Jak2 signaling, within the CD34-expressing endothelium and an intra-aortic cluster of the E11.5 AGM (arrows). The first HSCs are thought to emerge within these clusters. Professional illustration by Patrick Lane, ScEYEnce Studios.
This panel adapted from Mascarenhas et al depicts the expression of total Stat5, which is activated by Jak2 signaling, within the CD34-expressing endothelium and an intra-aortic cluster of the E11.5 AGM (arrows). The first HSCs are thought to emerge within these clusters. Professional illustration by Patrick Lane, ScEYEnce Studios.
Blood formation first begins in the extraembryonic tissues of the yolk sac; between embryonic days (E)7.5 and E10.5, primitive erythroid cells, the first myeloid cells, and multipotent progenitor cells (also known by their operational designation colony-forming units culture [CFU-C]) are produced. The journey of the adult HSC lineage begins within a transient structure located in the caudal half of the embryo proper known as the aorta-gonad-mesonephros (AGM) region. HSCs first emerge in the AGM region between E10.5 and E11.5 and subsequently undergo a series of spatial transitions that culminate in seeding of the fetal bone marrow in the days preceding birth,2 so laying the foundation of the adult hematopoietic system.
The feature that distinguishes the E10.5 to E11.5 AGM region from other midgestation HSC-containing organs is its capacity to generate HSCs when maintained ex vivo, making the process of HSC formation amenable to interference or enhancement using exogenous factors. Exploiting this capacity, Mascarenhas et al investigate the effect of exposing explants of AGM region to either exogenous interleukin 3 (Il3) or thrombopoietin (Thpo), the receptors of which engage the Jak-signal transducer and activator of transcription (Stat) signaling pathway, which resulted in an increase in both HSC and CFU-C numbers. Chemical interference experiments demonstrated that Jak2 inhibition specifically impeded the proliferative effects of Il3, whereas phosphatidylinositol 3-kinase (Pi3k) inhibition neutralized both the effects of Il3 and Thpo stimulation. Furthermore, inhibition of both Pi3k and Jak2 dramatically ablated the CFU-C content of AGM region explants, although whether this resulted from CFU-C death rather than actively blocking proliferation remains unclear.
Following the observation that chemical inhibition of Jak2 blocks CFU-C expansion ex vivo, the authors investigated how loss of endogenous Jak2 affects hematopoietic development in vivo. Using a Jak2 knock-in mouse line that renders the endogenous allele nonfunctional, Mascarenhas et al demonstrated that, although CFU-C numbers were unperturbed by the loss of Jak2, HSC function was almost completely ablated. This finding is intriguing because although formation of the first HSC is accomplished in the AGM, many of the resident CFU-Cs are regarded to be of yolk sac origin. A reasonable conclusion from these experiments is that hematopoietic specification in the yolk sac can occur without Jak2-mediated signaling but that a new set of environmental response mechanisms (that are Jak2 dependent) are engaged during HSC formation (see figure).
The combination of ex vivo and in vivo experiments has allowed Mascarenhas et al to introduce Thpo as a potentially important functional player in the development of the HSC lineage. However, given that removing the Thpo receptor Mpl does not ablate HSC development in the AGM region in vivo,3 it remains to be clarified how important Thpo-Mpl signaling is under physiologic conditions. Perhaps activation of the downstream pathways of Jak2 signaling is what is necessary (via factors such as Il3 or Thpo), and the initiating ligand–receptor interaction is largely irrelevant.
Therefore, in a world in which the HSC state can be programmed from adult progenitor cells,4 why should we care about HSC formation in the embryo? The answer to this question is beautifully illustrated by experiments performed by Mascarenhas et al using the JAKV617F expression model. JAKV617F is an acquired mutation that occurs within the human population, producing a constitutively active Jak2 that drives the formation of myeloproliferative neoplasms (MPNs).5-9 The JAKV617F mouse model used in this study, which expresses JAKV617F under the regulatory control of the endogenous Jak2 locus, results in the onset of erythrocytosis and thrombocytosis within a number of weeks10 and can be effectively transferred via bone marrow transplantation.10 Mascarenhas et al found that if HSCs from the E11.5 AGM region were used as donor material, the expected pathology did not develop (no evidence of erythrocytosis or thrombocytosis was detected over a prolonged period of time and across 2 rounds of transplantation) and the DNA damage that characterizes adult JAKV617F cells was also absent. From these data, the authors concluded that even when exposed to the adult environment, HSCs of embryonic origin and their progeny remained resistant to the potent effects of JAKV617F, implying that some degree of imprinting occurred.
Although it remains unclear how the level of JAKV617F expression from embryonic HSC grafts compares with those of HSCs from adult bone marrow origin, this is an exciting observation with regard to the application of developmental hematopoiesis to personalized therapeutic translation (such as the induction of the HSC state from pluripotent cells or the direct reprogramming of adult material into HSCs): if embryonic-like HSCs can be generated in vitro, any of the unknown factors that predispose an individual to the JAKV617F mutation could be neutralized.
One critical question is why does JAKV617F not result in MPN development following embryonic HSC transplantation? Mascarenhas et al suggest one possible explanation: that higher levels of Socs3 (an effective suppressor of both wild-type and mutant Jak2) expression in the embryonic tissue effectively manages this genetic lesion. Although testing of this hypothesis will undoubtedly follow, this study highlights a facet of embryonic HSC transplantation into the adult environment that we are yet to understand: to what extent does the adult bone marrow niche alter the genomic landscape of the donor HSC? For the SOCS3-JAKV617F axis to hold true, one would predict that the embryonic donor HSC graft, including downstream progeny, would retain the higher level of SOCS3 expression. Understanding this and the broader changes (or lack thereof) that occur following engraftment of the embryonic HSC on the adult environment is surely the next critical step.
It will be fascinating to see which other pathologies induced by genetic lesions can be bypassed by using adult-like HSCs from the embryonic organism.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal