In this issue of Blood, Man et al demonstrate that the transforming activity of the leukemic fusion oncogene MLL-AF9 is regulated by inhibitor of DNA binding 1 (Id1), a dominant-negative regulator of E protein transcription factors.1
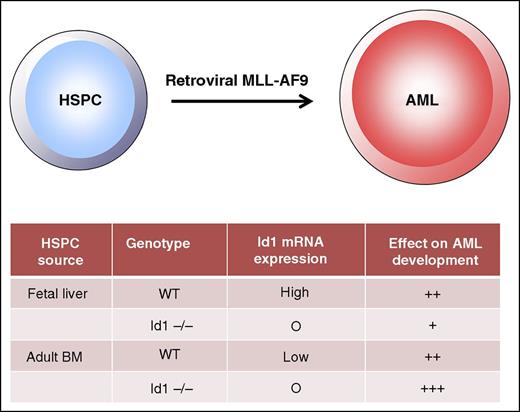
Fetal liver and adult mouse HSPCs exhibit different dependencies on Id1 for MLL-AF9–mediated leukemogenesis. Retroviral transduction of fetal liver HSPCs and adult bone marrow with MLL-AF9 induces acute myeloid leukemia (AML); however, deletion of Id1 in fetal liver HSPCs significantly attenuates leukemogenesis, whereas deletion of Id1 in adult bone marrow HSPCs promotes leukemic development. Despite these differences, in both cell contexts, p21, a target of Id1, mediates these effects.
Fetal liver and adult mouse HSPCs exhibit different dependencies on Id1 for MLL-AF9–mediated leukemogenesis. Retroviral transduction of fetal liver HSPCs and adult bone marrow with MLL-AF9 induces acute myeloid leukemia (AML); however, deletion of Id1 in fetal liver HSPCs significantly attenuates leukemogenesis, whereas deletion of Id1 in adult bone marrow HSPCs promotes leukemic development. Despite these differences, in both cell contexts, p21, a target of Id1, mediates these effects.
Although Id1 is not required for MLL-AF9–induced transformation, Id1 exerts a cell context–specific effect on this process, as loss of Id1 inhibits the development of MLL-AF9 leukemias initiated from fetal liver (FL) hematopoietic stem/progenitor cells (HSPCs), but enhances this process when leukemia is initiated from adult bone marrow HSPCs. The results from the adult HSPCs were particularly surprising because previous studies showed that Id1 is required to maintain adult hematopoietic stem cell (HSC) self-renewal and their undifferentiated state.2 Despite these cell-of-origin–specific effects of Id1, deletion of the Id1 target gene p21 was sufficient to rescue Id1-dependent phenotypes in leukemias initiated from both FL and adult HSPCs.
To further investigate the mechanisms by which Id1 exerts its effects on leukemogenesis, the authors measured Id1 expression and showed that FL HSPCs express higher levels of Id1 mRNA than adult HSPCs (see figure). This finding suggests that FL HSPCs may be more dependent on Id1 for their self-renewal properties than adult HSCs and that loss of Id1 reduces their susceptibility to transformation and/or the self-renewal of the resulting leukemia stem cells. To determine whether such a dependency is established at the time of disease initiation or during progression, it would be important to delete or reduce Id1 expression following MLL-AF9 transduction. The effect of Id1 loss on leukemias generated from adult HSPCs is more difficult to explain, as adult HSCs exhibit reduced self-renewal and increased myeloid differentiation.2 However, one may hypothesize that the Id1 loss induces alterations in adult HSPC function that potentiate the leukemic effect of MLL-AF9 by promoting HSPC differentiation into the myeloid lineage. This possibility would be consistent with prior studies that have shown that culture conditions that promote myeloid differentiation during the short culture period following retroviral transduction may also alter the efficiency of transformation and lineage of leukemias generated from cord blood (CB) CD34+ cells.3,4
Previous studies have shown that MLL-AF9–induced transformation differs in immature and adult HSPCs, but these findings are model system dependent. In the MLL-AF9 knock-in mouse model, FL HSPCs exhibit increased latency and induce leukemias expressing both myeloid and lymphoid markers.5 In contrast, human CD34+ CB cells are easier to immortalize than adult CD34+ cells, and CB CD34+ cells generate both AML and acute lymphoblastic leukemia in vivo, whereas adult CD34+ cells give rise to long-term myeloid-biased grafts but not leukemia.6 Intriguingly, the expression of classical downstream targets of MLL-AF9 including HoxA9, Meis1, and others was not significantly different in MLL-AF9–expressing CD34+ CB and adult HSPC cells, leaving open the question of what other pathways may regulate these differences.6 Although the lineage differences in leukemias from FL and CB HSPCs vs adult HSPCs are thought to reflect cell-of-origin differences between MLL-AF9+ leukemia in infants and adults,7 it is not clear whether Id1 participates in determining these lineage differences because the MLL-AF9 retroviral model used in these studies gives rise exclusively to AML. Nonetheless, consistent with the predictions made by these mouse experiments regarding the importance of Id1 expression in infant vs adult-type leukemia, MLL-AF9+ leukemias from patients age of ≤3 years express higher levels of Id1 mRNA than patients >3 years of age.1
As noted by the authors, the paradoxical effects of Id1 on MLL-AF9 leukemogenesis based on the age of the HPSCs in which they arise raises the disconcerting possibility that infant and adult AMLs driven by the same initiating mutation may exhibit different responses to drugs targeting the same pathway, particularly in those that might be developmentally regulated. Given that intrinsic differences in the cell-of-origin may determine the susceptibility of genetically identical leukemias to therapies, it will be important to extend these studies of Id1 to other models of leukemia that occur in both young and old patients, as well as confirm these findings using primary MLL-AF9+ patient samples, as well as genetically faithful MLL-AF9 human models. Given the recent demonstration that MLL-AF9–driven leukemias can be generated in human CB CD34+ cells using gene-editing strategies,8 it may be possible to perform the latter experiments manipulating Id1 expression in adult CD34+ cells expressing physiologic levels of MLL-AF9, thereby avoiding potential confounding factors associated with retroviral models of MLL-AF9+ leukemia that may not recapitulate all the features of human disease.9
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal