In this issue of Blood, Sepulveda and colleagues show that heterozygous mutations in different hemophagocytic lymphohistiocytosis (HLH)–associated genes add up, thereby conferring increased risk of developing disease.1 A fraction of HLH patients are explained by autosomal recessive mutations in genes required for lymphocyte cytotoxicity.
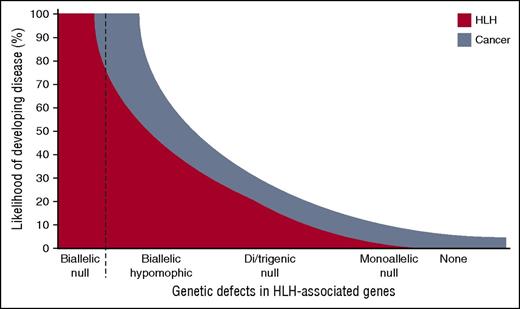
Schematic representation of the risk for developing HLH in relation to different models of genetic deficiency. Individuals with biallelic null mutations invariably develop HLH early in life, whereas hypomorphic mutations can be associated with late-onset HLH or cancer. Recently, it was suggested that monoallelic null mutations may represent a risk factor for developing cancer. Here, Sepulveda and colleagues elegantly show that polygenic monoallelic mutations add up, being associated with an intermediate risk of developing HLH. Likely, such polygenic combinations of mutations also increase the risk of cancer.
Schematic representation of the risk for developing HLH in relation to different models of genetic deficiency. Individuals with biallelic null mutations invariably develop HLH early in life, whereas hypomorphic mutations can be associated with late-onset HLH or cancer. Recently, it was suggested that monoallelic null mutations may represent a risk factor for developing cancer. Here, Sepulveda and colleagues elegantly show that polygenic monoallelic mutations add up, being associated with an intermediate risk of developing HLH. Likely, such polygenic combinations of mutations also increase the risk of cancer.
HLH is a rare, often-fatal syndrome with prolonged fever and hepatosplenomegaly as cardinal symptoms. These symptoms, combined with laboratory findings of cytopenias, elevated ferritin, hypertriglyceridemia, and hypofibrinogenemia, represent diagnostic criteria for HLH.2 In addition, liver failure and neurological manifestations are common features. Studies of familial cases have revealed impaired lymphocyte killing of target cells as an important cause of HLH. Autosomal recessive null mutations in PRF1, UNC13D, STXBP2, STX11, RAB27A, and LYST abolish target cell killing mediated by cytotoxic T lymphocytes and natural killer cells and are invariably associated with manifestation of HLH in childhood.3 Depending on the affected gene, significant differences exist in the average age at onset. Furthermore, individuals with biallelic hypomorphic mutations in such genes typically develop HLH later in life or, alternatively, present with hematological malignancies.4 Findings of biallelic mutations in HLH-associated genes warrant hematopoietic stem cell transplantation, which currently represents the only cure for inherited, so-called “primary” HLH. Importantly, many HLH patients, mostly with a later onset yet poor clinical prognosis, are not explained by current genetic insights. Such “secondary” HLH is typically associated with certain pathogen infections, hematological malignancies, or autoimmune conditions. A proportion of such secondary HLH patients carry a heterozygous mutation in HLH-associated genes.5 Intriguingly, 2 or more heterozygous mutations in distinct HLH-associated genes have been described in some patients.6 Whether the accumulation of monoallelic mutations in different genes can increase the risk of developing HLH is not known.
Capitalizing on different mouse models of primary HLH, Sepulveda and colleagues determined whether multiple monoallelic mutations in distinct genes required for lymphocyte cytotoxicity could contribute to the development of HLH.7 Crossing mice with mutations in Prf1, Stx11, or Rab27a, they generated animals carrying combinations of 2 or 3 heterozygous mutations. Using an established mouse model of primary HLH, animals were challenged with lymphocytic choriomeningitis virus (LCMV). In Prf1-deficient mice, LCMV infection causes fatal, T cell–driven, HLH-like disease within 2 weeks of infection.7 Sepulveda and colleagues monitored several clinical parameters of HLH disease activity as well as cytotoxic lymphocyte function. Whereas mice with heterozygous mutations in Prf1, Stx11, or Rab27a did not develop any overt phenotype upon LCMV infection, 20% and 30% of mice carrying double Prf1 and Rab27a or triple Prf1, Stx11, and Rab27a heterozygous mutations, respectively, succumbed to infection. Notably, mice carrying double Stx11 and Rab27a heterozygous mutations did not succumb, in line with a relatively reduced severity of HLH in Stx11-deficient mice. With respect to anemia, decreased platelet counts, and liver enzymes, mice with double and triple mutations generally displayed intermediate values, relative to Prf1-deficient animals that all died. In immunological terms, double and triple mutations amounted to increased serum levels of interferon-γ, interleukin-6, and tumor necrosis factor, representing cytokines that mediate pathology in HLH. Notably, cytotoxic granule trafficking and exocytosis was reduced in mice carrying heterozygous mutations in both Stx11, and Rab27a, but only to 2/3 of wild-type mouse levels. In summary, Sepulveda and colleagues thus found that an accumulation of heterozygous mutations in HLH-associated genes in mice correlated with transient HLH manifestations following viral infection and a fatal outcome in a small proportion of animals. The number and severity of disease features thus correlated with gene dosage.
The findings by de Saint Basile and coworkers3 have several implications for human disease. Their study supports the concept of a polygenic inheritance of HLH, where accumulation of partial genetic defects in cytotoxic granule-dependent pathway may increase the likelihood of developing disease (see figure). Of note, in a cohort of 2701 patients with a clinically suspected diagnosis of HLH, Zhang and colleagues found that only 1% of patients carried heterozygous mutations in 2 or more different HLH-associated genes.6 Because noncoding aberrations represent a significant cause of HLH that were not assessed in the study by Zhang et al, this figure likely represents an underestimate.6,7 In individual cases, calculating the risk of HLH based on genetic information is complicated. First, the effect of a specific mutation is not easily quantified. Distinct HLH-associated genes are associated with different severities. Some mutations may even display dominant negative effects, which are determined through advanced cellular assays.8 Second, with less severe impairments in lymphocyte cytotoxicity, environmental factors play an increasing role in determining HLH susceptibility.3 Nonetheless, the concept of gene dosages in determining the efficacy of target cell killing may have wider implications for the risk of developing a spectrum of diseases associated with impaired lymphocyte cytotoxicity. As the authors point out,1 lymphocyte cytotoxicity gene dosages may combine with mutations in genes regulating inflammation, thereby contributing to the risk of developing inflammatory syndromes such as macrophage activation syndrome. Moreover, biallelic mutations in HLH-associated genes have been linked to cancer susceptibility.4 Relatives of primary HLH patients, likely carriers of heterozygous mutations, display a 3-fold increased risk of cancer, specifically in female carriers.9 It is therefore conceivable that an accumulation of genetic lesions in the molecular pathway required for lymphocyte cytotoxicity may substantially increase the risk of developing cancer (see figure). Large-scale genome projects have revealed that 16% of the human population carries at least 1 predicted damaging mutation in a gene required for lymphocyte cytotoxicity.10 Accumulation of mutations in such genes may therefore signify an important mechanism for HLH as well as the spectrum of related diseases in a relatively large number of individuals.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal