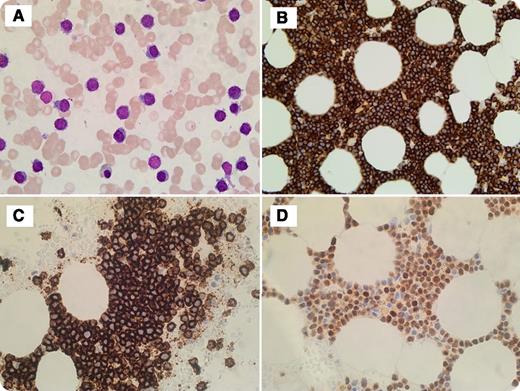
A 78-year-old man with a previous history of prostate cancer and cholecystectomy presented to the clinic with increased fatigue and shortness of breath upon exertion. Blood work showed a hemoglobin level of 77 g/dL, a white blood cell count of 5.8 × 109/L, a platelet count of 67 × 109/L, and immunoglobulin G κ paraproteins. Imaging study showed no lytic lesions. Bone marrow study showed diffuse involvement by sheets of lymphoid-like cells. Morphologically, cells were small, with lymphoplasmacytic features without prominent nucleoli (bone marrow aspirate, panel A). Immunohistochemistry showed these cells strongly positive for CD138, CD20, and cyclin D1 (panels B-D, respectively). Flow cytometry analysis demonstrated an abnormal population of cells positive for CD138/CD38, CD20 (bright), and CD56 with cytoplasmic κ restriction, and cells negative for CD19 and CD117. Fluorescence in situ hybridization study detected immunoglobulin heavy locus (IGH)-CCND1 rearrangement, monosomy13/13q deletion, and absence of IGH-FGFR3, IGH-MAF rearrangements, as well as 17p (TP53) deletions. The diagnosis of plasma cell myeloma was rendered.
This case highlights the rare lymphoplasmacytic morphology of plasma cells with cyclin D1 overexpression. Some studies suggest that this t(11;14) translocation with CD20 expression carries a better overall survival compared with those without CD20 expression. Knowing this morphologic variant of plasma cell neoplasm is important in differentiating it from other cyclin D1–positive lymphoproliferative disorders such as mantle cell lymphoma, B-cell chronic lymphocytic leukemia, hairy cell leukemia, and splenic lymphoma with villous lymphocytes.
A 78-year-old man with a previous history of prostate cancer and cholecystectomy presented to the clinic with increased fatigue and shortness of breath upon exertion. Blood work showed a hemoglobin level of 77 g/dL, a white blood cell count of 5.8 × 109/L, a platelet count of 67 × 109/L, and immunoglobulin G κ paraproteins. Imaging study showed no lytic lesions. Bone marrow study showed diffuse involvement by sheets of lymphoid-like cells. Morphologically, cells were small, with lymphoplasmacytic features without prominent nucleoli (bone marrow aspirate, panel A). Immunohistochemistry showed these cells strongly positive for CD138, CD20, and cyclin D1 (panels B-D, respectively). Flow cytometry analysis demonstrated an abnormal population of cells positive for CD138/CD38, CD20 (bright), and CD56 with cytoplasmic κ restriction, and cells negative for CD19 and CD117. Fluorescence in situ hybridization study detected immunoglobulin heavy locus (IGH)-CCND1 rearrangement, monosomy13/13q deletion, and absence of IGH-FGFR3, IGH-MAF rearrangements, as well as 17p (TP53) deletions. The diagnosis of plasma cell myeloma was rendered.
This case highlights the rare lymphoplasmacytic morphology of plasma cells with cyclin D1 overexpression. Some studies suggest that this t(11;14) translocation with CD20 expression carries a better overall survival compared with those without CD20 expression. Knowing this morphologic variant of plasma cell neoplasm is important in differentiating it from other cyclin D1–positive lymphoproliferative disorders such as mantle cell lymphoma, B-cell chronic lymphocytic leukemia, hairy cell leukemia, and splenic lymphoma with villous lymphocytes.
For additional images, visit the ASH IMAGE BANK, a reference and teaching tool that is continually updated with new atlas and case study images. For more information visit http://imagebank.hematology.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal