Autologous hematopoietic stem cell transplantation (HSCT) is increasingly used for severe autoimmune and inflammatory diseases, but the mechanisms involved have yet to be elucidated. In this issue of Blood, Delemarre et al report their findings in both animal and human models which provide insights into restoration of functionality and diversity within the regulatory T-cell (Treg) compartment following HSCT.1
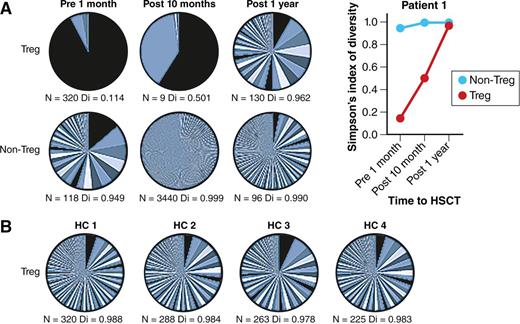
Successful autologous HSCT leads to a renewed and more diverse Treg TCR repertoire. (A) Patient with severe autoimmune disease pre- and postautologous HSCT. The pie charts show TCR β-chain sequencing results in Treg and non-Treg cells collected from a patient with severe autoimmune disease before and after autologous HSCT. N represents the number of different TCR sequences per sample and Di indicates diversity (0 = no diversity, 1 = maximal diversity). Changes in Di before and after HSCT are also shown in the graph. (B) Four healthy controls (HC). For comparison, TCR β-chain sequencing results in Treg cells from 4 HCs are shown in a similar fashion. The figure has been adapted from Figure 4 in the article by Delemarre et al that begins on page 91. Professional illustration by Patrick Lane, ScEYEnce Studios.
Successful autologous HSCT leads to a renewed and more diverse Treg TCR repertoire. (A) Patient with severe autoimmune disease pre- and postautologous HSCT. The pie charts show TCR β-chain sequencing results in Treg and non-Treg cells collected from a patient with severe autoimmune disease before and after autologous HSCT. N represents the number of different TCR sequences per sample and Di indicates diversity (0 = no diversity, 1 = maximal diversity). Changes in Di before and after HSCT are also shown in the graph. (B) Four healthy controls (HC). For comparison, TCR β-chain sequencing results in Treg cells from 4 HCs are shown in a similar fashion. The figure has been adapted from Figure 4 in the article by Delemarre et al that begins on page 91. Professional illustration by Patrick Lane, ScEYEnce Studios.
The findings of Delemarre et al come some 25 years after their fellow Dutch investigator, the late Prof Dirk van Bekkum (1925-2015), started to lay down the preclinical foundations for using HSCT in autoimmune diseases with an elegant series of experiments.2,3 Over the last 2 decades the field has developed gradually in the clinical setting in a variety of diseases. International databases now have several thousand patients and there is an increasing evidence base of large series and randomized controlled trials to support the use of autologous HSCT, particularly in multiple sclerosis (MS), connective tissue diseases, and Crohn’s disease. Consensus clinical guidelines are now available to assist with patient selection and treatment schedules.4,5
To date, a variety of mechanisms have been proposed to explain the clinical effects (and associated immune “reboot”) of autologous HSCT in severe autoimmune diseases. Whereas a “debulking of inflammation” is an instantaneous and predictable effect of any high-dose cytotoxic conditioning regimen, sustained clinical responses are best explained by long-term alterations in immune reconstitution via thymic and/or extrathymic pathways. Shifts in T- and B-cell subpopulations from memory to naive cell dominance, with restoration of polyclonal T-cell receptor (TCR) diversity, correction of immune gene expression abnormalities, and other changes in T cells, B cells, plasmablasts, and natural killer cells support immune re-education and tolerization with autologous HSCT. Like clinical responses, the ability to reconstitute varies between diseases and patients.5-10 However, 1 finding common to several diseases, including MS, systemic lupus erythematosus, systemic sclerosis, and juvenile idiopathic arthritis, is enhanced posttransplant levels of Treg cells.5,6,8-10
Delemarre and coworkers provide a further step toward a better understanding of the resetting of the Treg compartment. First, they report the reconstitution of host and donor-derived Treg compartments following congenic HSCT in a proteoglycan-induced mouse arthritis model. Although initially both populations expand, the naive, donor-derived Treg pool predominates to reconstitute a stable, functional, and tolerizing donor-derived Treg compartment.
Second, they use baseline and follow-up samples taken from pediatric patients receiving autologous HSCT for juvenile idiopathic arthritis and dermatomyositis. Prior to HSCT, patients displayed characteristics of a restricted oligoclonal Treg receptor repertoire. Subsequent clinical improvements (with 1 patient out to more than a decade) were associated with a striking resetting of Treg TCR diversity. TCR diversity of other non-Treg compartments also increased, although this was nowhere near as pronounced as changes to the Treg compartment (see figure).
Finally, returning to their animal model, they attempt to augment the therapeutic effect with additional Treg infusions. However, the biology in this area is clearly not so straightforward. Despite decreasing proinflammatory cytokine production, there was none of the desired improvement in clinical indices. Instead, there was a delay in immune reconstitution of the graft-derived T-cell compartment and, in view of the potential for risk, the authors highlight caution in extrapolating a similar approach for clinical studies.
What implications does this study have for other diseases? Clearly, autoimmune diseases are diverse in both their etiologies, pathogenesis, and manifestations, and the clinical studies were performed in relatively rare pediatric diseases. Similar studies are warranted in autoimmune diseases that are more commonly transplanted, such as MS, systemic sclerosis, and Crohn’s disease, and across all age groups given the variation in thymic involution and rebound.
Response to autologous HSCT also varies within the same disease and has been related to patient-related factors, such as stage of disease, and there is scope for improving responses and selecting the best patients. In MS, restoration of TCR diversity has been reported as a potential predictor of clinical response.9 Outcomes in juvenile idiopathic arthritis have also been related to the degree of thymic processing in reconstituting the T-cell compartment.10 TCR characterization may enable monitoring of pathogenic or protective T-cell clones following autologous HSCT for autoimmune diseases. Whether more specific assessment of Treg TCR diversity, as described by Delemarre et al, is a better predictor remains to be established. It is possible that such testing may assist with the selection of patients most likely to respond from HSCT and in identifying patients who may benefit from early interventions, including low-dose maintenance or salvage treatments or additional cellular therapies. However, in the current study, despite the reasonable logic, no benefit was achieved through additional Treg infusions. Clearly, this area requires more investigation.
Mechanistic studies may have been historically challenging to coordinate across a range of autoimmune diseases, perhaps because autologous HSCT has most often been used sporadically as an exceptional treatment in refractory patients, and sometimes as an emergency salvage procedure. With a greater evidence base the numbers of patients being treated are increasing substantially in some diseases, such as MS. Moreover, patients are being treated at an earlier stage in their disease, when both end organ damage and immune defects are potentially more reversible. Recent publication of international biobanking guidelines may facilitate access of samples to laboratories for specialized immune reconstitution studies before and after HSCT.6
Destroying dysfunctional immune systems with autologous HSCT and then closely observing how they are rebuilt from scratch via thymic and other pathways not only helps us to ameliorate intractable disabling and life-threatening states, but also has also provided useful insights into the pathogenesis of autoimmune diseases. By highlighting the ability of autologous HSCT to generate a naive, functional, and diverse donor-derived Treg compartment, Delemarre and colleagues add a further piece to van Bekkum’s visionary jigsaw.
Conflict-of-interest disclosure: The author declares no competing financial interests.