Abstract
Hypereosinophilic syndromes (HESs) are a group of rare disorders characterized by peripheral blood eosinophilia of 1.5 × 109/L or higher and evidence of end organ manifestations attributable to the eosinophilia and not otherwise explained in the clinical setting. HESs are pleomorphic in clinical presentation and can be idiopathic or associated with a variety of underlying conditions, including allergic, rheumatologic, infectious, and neoplastic disorders. Moreover, the etiology of the eosinophilia in HESs can be primary (myeloid), secondary (lymphocyte-driven), or unknown. Although corticosteroids remain the first-line therapy for most forms of HESs, the availability of an increasing number of novel therapeutic agents, including tyrosine kinase inhibitors and monoclonal antibodies, has necessarily altered the approach to treatment of HESs. This review presents an updated treatment-based approach to the classification of patients with presumed HES and discusses the roles of conventional and novel agents in the management of these patients.
Definitions
Mild blood eosinophilia, as defined by an absolute eosinophil count (AEC) between 0.5 and 1.0 × 109/L, is common, occurring in 3% to 10% of individuals depending on the population studied.1,2 Frequent causes include atopic disease, asthma, drug hypersensitivity, and helminth infection. In contrast, blood hypereosinophilia (HE), defined as an AEC of ≥1.5 × 109/L, is relatively rare and should prompt a thorough evaluation for an underlying cause (Table 1) and for evidence of end organ manifestations attributable to the eosinophilia, the defining feature of hypereosinophilic syndromes (HESs). Tissue HE is defined as (1) eosinophils >20% of all nucleated cells in a bone marrow aspirate; (2) tissue infiltration by eosinophils that, in the opinion of an experienced pathologist, is markedly increased; or (3) extensive extracellular deposition of eosinophil-derived proteins in tissue as demonstrated by immunostaining.3
Differential diagnosis of hypereosinophilia
| Category . | Examples (not inclusive) . |
|---|---|
| Allergic disorders* | Asthma, atopic dermatitis |
| Drug hypersensitivity | Varied† |
| Infection | |
| Helminthic | Varied, including strongyloidiasis, trichinellosis, filariasis, schistosomiasis, hookworm |
| Ectoparasite | Scabies, myiasis |
| Protozoan | Isosporiasis, sarcocystis myositis |
| Fungal | Coccidiomycosis, allergic bronchopulmonary aspergillosis, histoplasmosis |
| Viral | HIV |
| Neoplasms | Leukemia, lymphoma, adenocarcinoma |
| Immunologic disorders‡ | |
| Immunodeficiency | DOCK8 deficiency, Hyper-IgE syndrome, Omenn’s syndrome |
| Autoimmune and idiopathic | Sarcoidosis, inflammatory bowel disease, IgG4 disease, and other connective tissue disorders |
| Miscellaneous | Radiation exposure, cholesterol emboli, hypoadrenalism, IL-2 therapy |
| Rare eosinophilic disorders | Idiopathic hypereosinophilic syndrome, eosinophilic granulomatosis with polyangiitis (formerly Churg-Strauss syndrome), eosinophilic gastrointestinal disorders |
| Category . | Examples (not inclusive) . |
|---|---|
| Allergic disorders* | Asthma, atopic dermatitis |
| Drug hypersensitivity | Varied† |
| Infection | |
| Helminthic | Varied, including strongyloidiasis, trichinellosis, filariasis, schistosomiasis, hookworm |
| Ectoparasite | Scabies, myiasis |
| Protozoan | Isosporiasis, sarcocystis myositis |
| Fungal | Coccidiomycosis, allergic bronchopulmonary aspergillosis, histoplasmosis |
| Viral | HIV |
| Neoplasms | Leukemia, lymphoma, adenocarcinoma |
| Immunologic disorders‡ | |
| Immunodeficiency | DOCK8 deficiency, Hyper-IgE syndrome, Omenn’s syndrome |
| Autoimmune and idiopathic | Sarcoidosis, inflammatory bowel disease, IgG4 disease, and other connective tissue disorders |
| Miscellaneous | Radiation exposure, cholesterol emboli, hypoadrenalism, IL-2 therapy |
| Rare eosinophilic disorders | Idiopathic hypereosinophilic syndrome, eosinophilic granulomatosis with polyangiitis (formerly Churg-Strauss syndrome), eosinophilic gastrointestinal disorders |
Allergic disorders, including asthma and atopic dermatitis, can be associated with HE (AEC ≥1.5 × 109/L), especially in children, although extremely high eosinophil counts (AEC ≥5.0 × 109/L) should prompt consideration of another cause. Because allergic manifestations are common in patients with idiopathic HES and L-HES, the distinction between allergic disease with marked eosinophilia and HES with concomitant allergic disease may be impossible in some cases.
Drug hypersensitivity can occur in response to any prescription or nonprescription drug or supplement. Although drug-associated eosinophilia can be asymptomatic, well-described syndromes include eosinophilia-myalgia syndrome, drug reaction with eosinophilia and systemic symptoms, interstitial nephritis, and eosinophilic hepatitis.
HE can occur in the setting of a wide variety of immunologic disorders, particularly those characterized by dysregulation of lymphocyte proliferation or function. Signs and symptoms attributable to the eosinophilia may or may not be present and can be difficult at times to distinguish from manifestations of the underlying disorder.
The use of the term HES has evolved over the last 40 years since its first use by Hardy and Anderson to describe 3 patients with marked eosinophilia and eosinophilic cardiopulmonary involvement.4 Not only have improved diagnostic techniques led to the identification of previously unrecognized causes of HES, but the availability of effective therapies has led to a marked decrease in morbidity and mortality in patients with HES who are treated early (before the development of irreversible complications). In an attempt to address these issues, updated definitions and classification systems for HES have been proposed by the World Health Organization (WHO),5 consensus panels,3 and other experts6 (supplemental Table 1 available on the Blood Web site). Two major controversies remain: whether to include eosinophilic disorders of known etiology in the broad classification of HES and, if so, which disorders to include and how to define eosinophilic end organ damage.
For the purposes of this review, HES will be defined broadly as blood HE (AEC of ≥1.5 × 109/L) and clinical manifestations attributable to eosinophilia or tissue HE with blood eosinophilia (AEC above the upper limit of normal for the reference laboratory). Eosinophilic disorders of known cause, such as platelet-derived growth factor receptor α–associated myeloproliferative neoplasms (PDGFRA-associated MPNs)7 and eosinophilic endomyocardial fibrosis caused by helminth infection,8 are included in this definition of HES to emphasize the point that the clinical manifestations in these settings can be indistinguishable from those of idiopathic HES.
Case
A 42-year-old previously healthy woman presented to the emergency room with a 2-month history of worsening fatigue, weight gain, dyspnea on exertion, nonproductive cough, and orthopnea. She was afebrile, tachypneic, and tachycardic. Physical examination was notable for right lower lobe crackles and 1+ peripheral edema bilaterally. Labs revealed a leukocytosis of 15.7 × 109/L with 51% eosinophils (AEC, 8.0 × 109/L). A right lower lobe infiltrate was present on chest x-ray. Echocardiography showed global hypokinesis with a decreased ejection fraction of 40% and left apical hypertrophy without thrombus. Serum troponin was not measured.
Initial approach to the patient with HES
HE of any etiology can cause serious, potentially life-threatening, complications, including cardiac involvement, thromboembolism, and neurologic manifestations. Although clinical and laboratory findings associated with aggressive disease and poor prognosis include AEC > 100.0 × 109/L, signs of congestive heart failure, features suggestive of a myeloproliferative neoplasm (eg, splenomegaly, presence of early myeloid precursors on the peripheral smear, elevated serum B12, and/or tryptase levels), and resistance to corticosteroid therapy,9-11 specific biomarkers of disease progression have not been identified to date. Consequently, the decision to initiate urgent therapy depends on both the acuity and severity of the clinical presentation and the perceived risk of rapid progression.
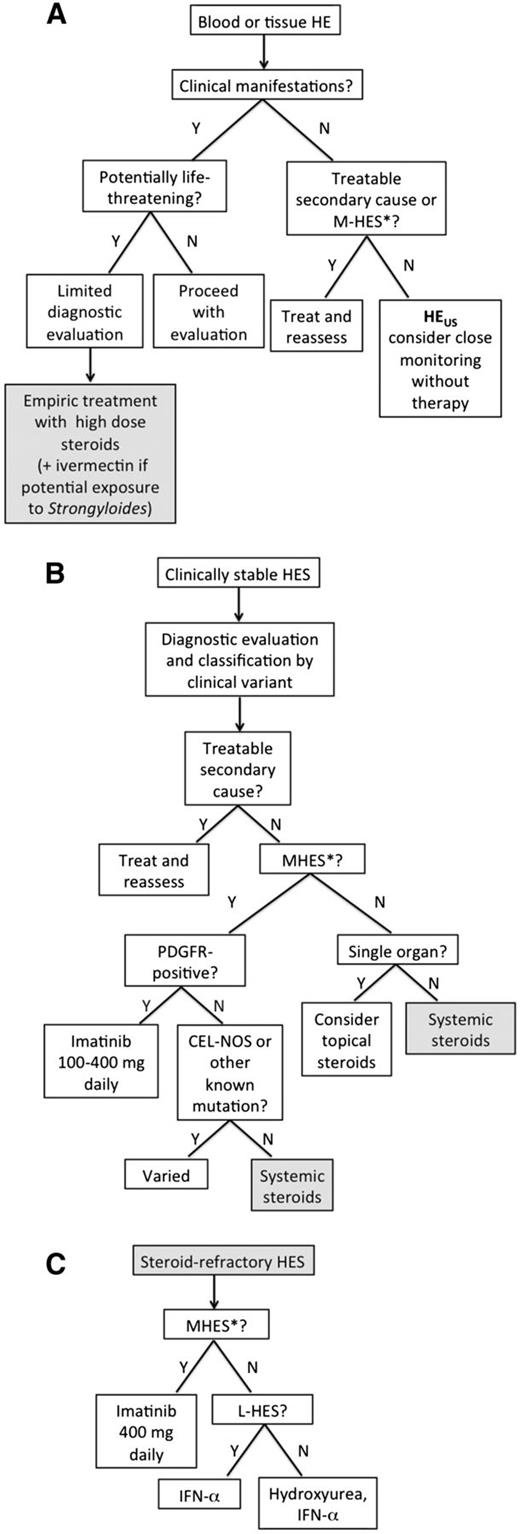
When life-threatening manifestations are present or imminent, as in the case presented, high-dose corticosteroid therapy should be initiated immediately. Recommended dosing ranges from 1 mg/kg prednisone to 1 g methylprednisolone depending on the severity of the clinical manifestations. Intravenous dosing should be considered to ensure adequate absorption in patients who are acutely ill or have signs or symptoms of gastrointestinal involvement (Figure 1). Patients with a history of potential exposure to Strongyloides should receive concomitant empiric ivermectin therapy (200 μg/kg orally daily for 2 days) to prevent corticosteroid-associated hyperinfection syndrome.12 Although every effort should be made to obtain appropriate diagnostic studies (Table 2) before initiating corticosteroid therapy, treatment should not be delayed in the face of worsening signs and symptoms.
Treatment-based approach to HESs. Algorithms are proposed for evaluation of (A) presumed HES, (B) clinically stable HES, and (C) steroid-resistant HES. *M-HES is defined for the purposes of this algorithm as HES with a genetic abnormality known to cause clonal eosinophilia or idiopathic HES with ≥4 of the following features: dysplastic eosinophils, serum B12 >737.8 pM (1000 pg/mL), serum tryptase >12 ng/mL, anemia and/or thrombocytopenia, splenomegaly, bone marrow cellularity >80%, myelofibrosis, spindle-shaped mast cells >25%, or strong clinical suspicion of a myeloproliferative disorder.
Treatment-based approach to HESs. Algorithms are proposed for evaluation of (A) presumed HES, (B) clinically stable HES, and (C) steroid-resistant HES. *M-HES is defined for the purposes of this algorithm as HES with a genetic abnormality known to cause clonal eosinophilia or idiopathic HES with ≥4 of the following features: dysplastic eosinophils, serum B12 >737.8 pM (1000 pg/mL), serum tryptase >12 ng/mL, anemia and/or thrombocytopenia, splenomegaly, bone marrow cellularity >80%, myelofibrosis, spindle-shaped mast cells >25%, or strong clinical suspicion of a myeloproliferative disorder.
Diagnostic studies
| Test . | Comment . |
|---|---|
| All patients with HES | |
| Complete blood count* | |
| Routine chemistries, including liver function tests* | |
| Quantitative serum immunoglobulin levels, including IgE | |
| Serum troponin*, echocardiogram | If abnormal, cardiac MRI should be considered as this may show characteristic features of eosinophilic involvement; tissue involvement may be patchy limiting the utility of biopsy |
| Pulmonary function tests* | |
| Chest/abdomen/pelvis CT* | To assess for splenomegaly, lymphadenopathy, and occult neoplasms |
| Bone marrow biopsy, including cytogenetics* | Recommended in all patients with AEC > 5.0 × 109/L and features of M-HES or L-HES. Should be considered in other patients |
| Biopsies of affected tissues (if possible)* | |
| Other testing as indicated by history, signs, and symptoms | Including parasitic serologies, anti-neutrophil cytoplasmic antibodies, and HIV |
| Serum tryptase and B12 levels | |
| FIP1L1/PDGFRA analysis by FISH or RT-PCR | Testing of peripheral blood is sufficient |
| T- and B-cell receptor rearrangement studies | |
| Lymphocyte phenotyping by flow cytometry* | At a minimum CD3, CD4, and CD8 and CD19 or 20 staining should be performed to assess for aberrant CD3−CD4+, CD3+CD4+CD8+, and CD3+CD4−CD8− populations and B-cell lymphoproliferative disorders |
| Patients with features of M-HES | |
| Additional testing for BCR-ABL1, PDGFRB, JAK2, FGFR1, and KIT mutations by PCR, FISH, or other methods, as appropriate | Testing should be guided by bone marrow findings |
| Patients with evidence of L-HES | |
| Consider PET scan,* lymph node biopsy* | |
| EBV viral load |
| Test . | Comment . |
|---|---|
| All patients with HES | |
| Complete blood count* | |
| Routine chemistries, including liver function tests* | |
| Quantitative serum immunoglobulin levels, including IgE | |
| Serum troponin*, echocardiogram | If abnormal, cardiac MRI should be considered as this may show characteristic features of eosinophilic involvement; tissue involvement may be patchy limiting the utility of biopsy |
| Pulmonary function tests* | |
| Chest/abdomen/pelvis CT* | To assess for splenomegaly, lymphadenopathy, and occult neoplasms |
| Bone marrow biopsy, including cytogenetics* | Recommended in all patients with AEC > 5.0 × 109/L and features of M-HES or L-HES. Should be considered in other patients |
| Biopsies of affected tissues (if possible)* | |
| Other testing as indicated by history, signs, and symptoms | Including parasitic serologies, anti-neutrophil cytoplasmic antibodies, and HIV |
| Serum tryptase and B12 levels | |
| FIP1L1/PDGFRA analysis by FISH or RT-PCR | Testing of peripheral blood is sufficient |
| T- and B-cell receptor rearrangement studies | |
| Lymphocyte phenotyping by flow cytometry* | At a minimum CD3, CD4, and CD8 and CD19 or 20 staining should be performed to assess for aberrant CD3−CD4+, CD3+CD4+CD8+, and CD3+CD4−CD8− populations and B-cell lymphoproliferative disorders |
| Patients with features of M-HES | |
| Additional testing for BCR-ABL1, PDGFRB, JAK2, FGFR1, and KIT mutations by PCR, FISH, or other methods, as appropriate | Testing should be guided by bone marrow findings |
| Patients with evidence of L-HES | |
| Consider PET scan,* lymph node biopsy* | |
| EBV viral load |
Substantially affected by corticosteroid therapy.
If the eosinophil count and symptoms do not improve after 1 to 2 days of high-dose corticosteroid therapy, a second agent should be added to rapidly lower the eosinophil count. To maximize the chance of response, selection of second-line agents should be guided by the clinical presentation. For example, imatinib mesylate is most appropriate if myeloproliferative disease is suspected,10 but is unlikely to be effective in a patient with lymphocyte-driven HES. Conversely, cyclophosphamide is effective in eosinophilic vasculitis13 but would not be the treatment of choice for a patient with PDGFRA-associated MPN. Additional agents that have been used to rapidly lower counts in steroid-refractory patients include high-dose hydroxyurea, vincristine, and mepolizumab (humanized anti-interleukin [IL]-5 antibody).14 Once the patient is stabilized, evaluation should proceed as outlined below.
Case (continued)
The patient was treated with a single dose of methylprednisolone 1 g intravenously with resolution of the eosinophilia (AEC, 0.0 × 109/L). Seven days later, she was transferred to a tertiary care center on no therapy. At this point, she underwent additional evaluation including repeat echocardiogram, which now revealed a left ventricular thrombus and pulmonary hypertension, and an endomyocardial biopsy with no evidence of inflammation. Low-molecular-weight heparin was started, and hematology was consulted for a rising eosinophil count (AEC, 8.9 × 109/L). Bone marrow histology was hypercellular (60%) with 29% eosinophils, 3+ reticulin fibrosis, and >10% mast cells, of which >25% were spindle shaped. Karyotype was normal, and fluorescence in situ hybridization (FISH) studies were negative for FIP1L1-PDGFRA, BCR-ABL1, and TEL-PDGFRB. T-cell receptor and immunoglobulin (IgH) rearrangement studies showed a polyclonal pattern, and flow cytometry did not detect aberrant T- or B-cell populations. Serum B12 and tryptase levels were within normal limits. Methylprednisolone 1 g intravenously was administered, and prednisone 80 mg by mouth daily was started for presumed idiopathic HES.
Clinical HE/HES variants
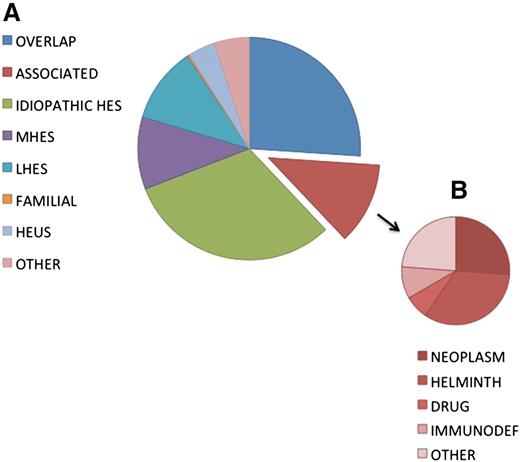
At a 2005 international consensus conference on HES treatment, a classification scheme was proposed that subdivided patients with HES into 6 clinically defined groups to facilitate treatment decisions.15 This classification was refined in 20106 and continues to be updated as our understanding of the relationship between etiology and response to therapy evolves.3 For the purposes of this review, HES will be divided into 6 clinical variants: myeloproliferative HE/HES (M-HE/M-HES), lymphocytic variant HE/HES (L-HE/L-HES), overlap HES, associated HE/HES, familial HE/HES, and idiopathic HES. HE of unknown significance (HEUS) will also be discussed. The relative frequencies of these variants in the general population are difficult to ascertain due in large part to the lack of universally accepted definitions of HES and referral bias (ie, a hematologist is more likely to see M-HES). Nevertheless, data from a multicenter retrospective study16 and from our cohort of 307 consecutive patients referred for unexplained HE (Figure 2) suggest that M-HES (including PDGFRA-associated MPN) and L-HES each account for 10% to 20% of patients with HES after treatable secondary causes are excluded.
Frequency distribution of diagnoses in a cohort of 302 subjects referred for evaluation of unexplained hypereosinophilia.
Frequency distribution of diagnoses in a cohort of 302 subjects referred for evaluation of unexplained hypereosinophilia.
Myeloproliferative HE/HES (M-HE or M-HES; HE or HES with documented or presumed clonal eosinophilic involvement)
Although it has long been recognized that HES is a “continuum of hypereosinophilic disease with eosinophilic leukemia existing at one pole,”4,9 the importance of identifying patients with M-HE/HES was not apparent until the tyrosine kinase inhibitor, imatinib mesylate, became available. Early studies demonstrated a dramatic response to imatinib in a subset of male patients with aggressive disease17 and features suggestive of a myeloproliferative process (eg, dysplastic eosinophils, circulating myeloid precursors, anemia, thrombocytopenia, splenomegaly, elevated serum B12 and/or tryptase levels, atypical mast cells).10 The majority of these imatinib-responders with HES were ultimately found to have an interstitial deletion in chromosome 4q12 causing the constitutively active fusion tyrosine kinase, FIP1L1-PDGFRA, which can be detected by FISH or reverse transcriptase-polymerase chain reaction (RT-PCR) in peripheral blood or bone marrow.7,18 Many other imatinib-sensitive PDGFR fusions, including KIF5B-PDGFRA and ETV6-PDGFRB,19-21 and point mutations in PDGFRA22 have been described in patients presenting with clinical features of HES, but are uncommon.
Clonal eosinophilia is present by definition in chronic eosinophilic leukemia-not otherwise specified (CEL-NOS) and can occur in patients with a variety of other myeloproliferative disorders, including D816V KIT-positive systemic mastocytosis23 and mixed lymphoid/myeloid neoplasms with rearrangements of FGFR1 or JAK2. End organ manifestations attributable to the eosinophilia may be present but are not universal. Treatment approaches vary depending on the underlying abnormality and will not be discussed in this review, although it is important to recognize that some mutations and chromosomal rearrangements associated with HES are not imatinib responsive24-26 and that novel inhibitors may be effective in some cases (eg, ruxolitinib has been reported to be effective in the treatment of 2 patients with PCM1-JAK2 who presented with eosinophilia27,28 ). Although rare patients with documented clonal abnormalities who are completely asymptomatic and without clinical manifestations (M-HE) may exist, there are no data in the literature to support withholding treatment in such cases. Consequently, they should be approached no differently than symptomatic patients with the same molecular or cytogenetic abnormality.
Finally, some patients who present with clinical and laboratory features that are indistinguishable from PDGFRA-associated MPN have no detectable cytogenetic or molecular abnormalities and do not meet criteria for acute leukemia or CEL-NOS. These patients with presumed clonal eosinophilia (or idiopathic M-HES) tend to have an aggressive course and may respond to imatinib (see below).
The above-described definition of M-HES is based predominantly on clinical and laboratory features that predict treatment response and prognosis within the broader context of patients presenting with HES (AEC of ≥1.5 × 109/L and clinical manifestations attributable to eosinophilia or tissue HE with blood eosinophilia). Molecular and cytogenetic abnormalities are incorporated when their presence alters these outcomes. This definition both differs from and overlaps with the current WHO guidelines, which categorize patients with myeloid and lymphoid neoplasms, including those with HES, on the basis of molecular, genetic, histopathologic, and selected clinical criteria.29 As such, patients with HES and myeloproliferative features may be classified among myeloid neoplasms (CEL-NOS, myeloid neoplasms associated with PDGFRA, PDGFRB, or FGFR1, atypical chronic myeloid leukemia), myeloproliferative disorders (myeloproliferative neoplasms unclassifiable, systemic mastocytosis with eosinophilia), or as idiopathic HES (supplemental Table 1).
Lymphocytic variant HE/HES (L-HE or L-HES; HE or HES with a demonstrable clonal or phenotypically aberrant lymphocyte population producing cytokines that drive eosinophilia)
The association between lymphoid malignancy and eosinophilia had been recognized for >50 years29 when the first patient with HES and expansion of a clonal CD3−CD4+ T-cell population secreting IL-5 was described.30 Since that time, several large series of L-HES patients have been reported.16,31-34 Although listed as an exclusion criteria for a diagnosis of idiopathic HES, L-HES is not otherwise classified in the 2008 WHO guidelines.5 Equally frequent in men and women, L-HES is characterized by a high prevalence of skin and soft tissue manifestations, elevated serum IgE, and thymus and activation regulated chemokine levels. L-HES typically has an indolent course, but may progress to overt lymphoma/leukemia in 5% to 25% of cases, sometimes after many years.33-35 The presence of an aberrant or clonal lymphocyte population in the absence of any clinical manifestations (L-HE) has been described is considered part of the spectrum of HEUS (see below).
Episodic angioedema and eosinophilia (EAE; Gleich’s syndrome) is a unique subset of L-HES in which patients have cyclic episodes of angioedema and urticaria that occur every 28 to 32 days and are accompanied by a rise in serum IL-5 levels and dramatic eosinophilia, all of which resolve without treatment between cycles.36,37 Although most (if not all) patients with EAE have a clonal CD3−CD4+ T-cell population, recent data suggest that EAE is a multilineage disorder, with cycling of eosinophils, neutrophils, and lymphocytes, as well as multiple cytokines and chemokines.38 Elevated serum IgM is also characteristic. Optimal treatment of this extremely rare disorder is unclear at this time.
Overlap HES (eosinophilic disease restricted to a single organ system accompanied by peripheral eosinophilia >1.5 × 109/L)
Whereas distinguishing single organ eosinophilic disorders, such as eosinophilic gastrointestinal disease, eosinophilic asthma, and eosinophilic dermatitis, from HES with multisystem involvement can be challenging in the presence of marked peripheral eosinophilia (hence the term “overlap HES”),39 the distinction has important consequences with respect to therapy. For example, although an elemental diet or swallowed fluticasone may be appropriate for a patient with isolated eosinophilic esophagitis irrespective of AEC,40,41 these therapies are unlikely to prevent progression of disease in multisystem HES.
Eosinophilic granulomatosis with polyangiitis (EGPA; Churg-Strauss syndrome) is a special category of overlap HES in which the underlying pathophysiology is related to eosinophilic infiltration of a single organ, namely the walls of small and medium blood vessels, but this results in multisystem involvement that presents with clinical and laboratory features that are often indistinguishable from those of HES with asthma and/or sinusitis.42 This is particularly true for the 30% to 40% of EGPA patients who do not have detectable levels of anti-neutrophil cytoplasmic antibodies.43 Whereas glucocorticoid therapy is recommended for the initial treatment of both EGPA and PDGFRA-negative HES, the recommended dose and duration of glucocorticoids and choices of second-line therapies differ.44
Associated HE/HES (eosinophilia >1.5 × 109/L in the setting of a distinct diagnosis, in which eosinophilia has been described in a subset of affected patients)
HE/HES can complicate a wide variety of clinical diagnoses. In some cases, such as helminth infection, the etiology can be readily identified and treatment is targeted at the underlying cause with no direct effect on the eosinophilia. Resolution of HE/HES in such instances confirms that the eosinophilia was a secondary phenomenon. In other cases, such as sarcoidosis45 or IgG4-related disease,46 the diagnosis is based on characteristic clinical features and pathologic findings, but therapy involves corticosteroids or other agents that also have activity against eosinophils blurring the distinction between associated and idiopathic HES.
Familial HE/HES
Familial forms of some eosinophilic disorders, such as eosinophilic esophagitis, are relatively common and involve environmental and genetic factors.47 In contrast, familial multisystem HES appears to be extremely rare. Autosomal dominant transmission of HE has been mapped to chromosome 5q31-33 in one extended family.48 Of note, although 2 members of the family developed HES with fatal endomyocardial fibrosis and neuropathy, most affected family members have remained asymptomatic despite lifelong HE.49
Idiopathic HES
As illustrated by the case presentation, after comprehensive evaluation, >50% of patients with HES are unable to be classified into one of the categories above and are considered idiopathic (Figure 2).
Hypereosinophilia of unknown significance (HEUS) is the term used to describe patients with persistent HE without symptoms or evidence of end organ manifestations in the absence of treatment50,51 and is used to highlight differences in the approach to treatment between asymptomatic and symptomatic patients with HE (see below). Although the presence of clinical features and/or genetic abnormalities consistent with an eosinophilic myeloproliferative disorder precludes a diagnosis of HEUS, abnormal or clonal lymphocyte populations have been described in some patients with HEUS and do not appear to be associated with disease progression.50 A family history should be elicited in all suspected cases of HEUS (see above).
Case (continued)
As the prednisone dose was tapered, the patient became more symptomatic with dyspnea and lower extremity edema. AEC on prednisone 20 mg daily was 6.5 × 109/L. Repeat echocardiography and cardiac magnetic resonance imaging showed persistence of the left ventricular thrombus with fibrotic material now filling one-third of the cavity and moderate mitral regurgitation. Repeat bone marrow biopsy was unchanged. Eosinophilia and symptoms persisted despite sequential trials of imatinib mesylate (300 mg by mouth daily for 4 weeks) and interferon-α (3 million units subcutaneously daily for 4 weeks).
General approach to treatment
As described above, the first step in any treatment algorithm is to assess the need for urgent intervention. An equally important decision is whether there is a need for treatment at all (Figure 1). This can be a difficult decision, because biomarkers that predict disease progression in patients with asymptomatic PDGFRA-negative HE (HEUS) are lacking.50,51 All patients with suspected HEUS should undergo evaluation for treatable secondary causes of HE, including drug hypersensitivity and helminth infection, as well as assessment for FIP1L1-PDGFRA and features suggestive of M-HES, because these patients require treatment to prevent disease progression. Additional factors to consider in the decision to withhold treatment in an individual patient with HEUS include the duration and degree of eosinophilia before evaluation for potential HES, likelihood of compliance with frequent monitoring, and risk factors for complications of chronic steroid use. Patients with HEUS who opt to forgo treatment should be monitored closely (at a minimum, every 3 months for the first 1 to 2 years) for the development of clinical manifestations of HES.
Once the decision has been taken to proceed with treatment, classification by clinical variant should be used to guide therapy (Figure 1). Because systemic corticosteroids remain the first-line therapy for most forms of HES, identification of patients with (1) secondary eosinophilia requiring specific therapy directed at the underlying etiology (associated HES), (2) PDGFRA-positive MPN or other steroid-resistant eosinophilic myeloproliferative disorders, and (3) overlap syndromes that can be managed with topical corticosteroid therapy should be a priority. The distinction between L-HES, idiopathic HES, and systemic forms of overlap HES becomes an important factor to consider in the choice of second-line therapies when steroid resistance or intolerance develops (Figure 1).
Associated HES
The most common secondary eosinophilic conditions requiring specific therapy are drug hypersensitivity, parasitic helminth infection, neoplasia (including lymphoma), and, in children, immunodeficiency disorders. Although a detailed discussion of the many and varied causes of secondary HE/HES is beyond the scope of this review and can be found in the published literature,52,53 a few points deserve mention. First, a detailed medical history is paramount, as it can provide valuable clues to the underlying diagnosis. This should include a complete list of all prescription and nonprescription drugs and supplements taken in the months preceding the onset of eosinophilia, recent and remote travel and exposures, family history of eosinophilic disorders, and a complete review of systems. Second, the time course of resolution of eosinophilia after successful treatment of secondary causes is variable. In fact, eosinophilia may resolve within days or persist for months after discontinuation of an offending drug,54,55 and transient exacerbation of eosinophilia is often seen following successful treatment of helminth infections.56 Finally, lack of response to seemingly appropriate treatment should prompt assessment for other causes of HES.
PDGFR-associated MPN
Patients with documented rearrangements or mutations involving PDGFRA should be treated with imatinib mesylate (100-400 mg by mouth daily). Corticosteroids (≥1 mg/kg prednisone or equivalent) should be administered during the first few days of imatinib therapy in patients with a history of cardiac involvement and/or elevated serum troponin levels to prevent myocardial necrosis, a rare complication of imatinib therapy in eosinophilic patients.57,58 The response to imatinib is typically within days to a few weeks, and complete hematologic and molecular remission is almost universal.7,58-62 Whereas maintenance of remission has been reported with imatinib doses as low as 100 mg weekly,63 daily dosing seems more prudent due to the theoretical risk of inducing resistance. Although early studies demonstrated relapse in all PDGFRA-positive patients within 2 to 3 months of imatinib discontinuation,64 recent data suggests that cure may be possible in some cases, especially after prolonged molecular remission.59,65 Because molecular relapse typically precedes recurrence of eosinophilia and clinical manifestations by several months,64 testing for the presence of FIP1L1-PDGFRA is recommended every 3 to 6 months in patients on a stable imatinib dose and every 3 months after drug discontinuation.
Imatinib resistance is uncommon in PDGFRA-associated MPN but has been reported.7,66-70 Several of the newer tyrosine kinase inhibitors (TKIs), including sorafenib and midostaurin, have in vitro activity against cells carrying the FIP1L1-PDGFRA fusion with the most common imatinib-resistant mutation (T674I), although only ponatinib has activity against the clinically relevant D842V mutation.71 Nevertheless, despite promising in vitro data, clinical data in imatinib-resistant HES are scarce. Successful use of nilotinib as primary therapy for M-HES has been reported in one case series,72 and both nilotinib and dasatinib have been used as salvage therapy in isolated patients with PDGFRA-associated MPN who were intolerant to imatinib.73,74 Sorafenib has been used in 2 patients with the imatinib-resistant T674I mutation with transient response,67,69 although outgrowth of a D842V-positive clone occurred in 1 case, ultimately leading to death of the patient.67 The use of other TKIs for imatinib resistant M-HES has not been reported to date. Patients with aggressive disease who progress despite TKI therapy should be considered for allogeneic hematopoietic stem cell transplantation.75
Imatinib is also first-line therapy for patients presenting with MPN due to rearrangements of PDGFRB, regardless of the fusion partner.76 Complete and durable remission was reported in >80% of subjects in a recent case series of 26 patients.77 Due to rarity of PDGFRB-associated MPN, there are only anecdotal or no data on imatinib dosing, resistance, or treatment interruption.
PDGFR-negative M-HES
High-dose corticosteroids are often effective in the short-term reduction of eosinophilia and clinical manifestations in patients with PDGFR-negative M-HES and are useful in the initial management of such patients.16 Unfortunately, many patients show only a transient or partial response and require treatment with additional agents. Although reported imatinib response rates in PDGFRA-negative HES vary widely (9-60%) depending on the series,16,78-80 recent data from our center suggest that the presence of myeloproliferative features (presumed clonal eosinophilic involvement) is an important predictor of imatinib response in patients with FIP1L1-PDGFRA–negative HES. Of note, PDGFR-negative patients often require higher doses of imatinib and appear to respond more slowly.79,81 Consequently, imatinib (400 mg daily for ≥4 weeks) is recommended. Patients experiencing a suboptimal or partial response should undergo repeat bone marrow examination, because unmasking of pre-B-cell acute lymphocytic leukemia has been reported in ≥1 patient with a partial response to imatinib.82 The most appropriate therapy for patients who fail steroid and imatinib therapy depends on the severity of the clinical manifestations. Possibilities include hydroxyurea, interferon-α, second- and third-generation TKIs, and allogeneic transplantation. Patients with aggressive disease and molecular or cytogenetic abnormalities that are typically resistant to steroid and imatinib therapy, including FGFR1 mutations, represent a special category of PDGFR-negative M-HES and should be considered early for alternative therapies.
Overlap HES
From a treatment perspective, patients with overlap HES can be divided into 2 groups: (1) patients with organ-restricted clinical manifestations who can often be managed with topical corticosteroids or other therapies directed specifically at the affected organ system41 and (2) patients with possible EGPA who require more aggressive therapy to prevent potentially life-threatening complications. Low to moderate doses of corticosteroids (ie, <20 mg prednisone daily) are often sufficient to control symptoms and prevent tissue fibrosis in the first group, whereas high-dose corticosteroid therapy with or without concomitant cytotoxic therapy is the cornerstone of therapy in the second group.44
Idiopathic HES
Corticosteroids remain the mainstay of therapy for idiopathic HES.14,16 Although high doses are effective in most patients, dosing and duration should be individualized based on the clinical manifestations, comorbidities, and perceived risk of serious end organ damage. Once the eosinophil count has normalized and symptoms have improved, the steroid dose should be tapered slowly with a goal of 10 mg prednisone equivalent or less daily. In patients who experience significant steroid side effects or who fail to respond adequately to therapy, as in the case presented, a second agent should be added. The most commonly used second-line therapies are hydroxyurea (1-2 g orally daily) and interferon-α (1-3 mU subcutaneously daily), each of which is effective in ∼30% of patients.14,16 Pegylated interferon has been used with equivalent results.83 Low-dose hydroxyurea (500 mg daily) has been reported to potentiate the effects of interferon-α without increasing toxicity in M-HES84,85 and is a reasonable alternative to escalating the interferon-α dose in HES patients who demonstrate partial response to interferon-α alone. Cyclosporine,16 alemtuzumab,86,87 and 2-chlorodeoxyadenosine88 have been used to treat small numbers of HES patients with some success but with considerable toxicity and are additional options for treatment-refractory patients. A variety of other agents have been used in the treatment of isolated cases of HES, but data are insufficient to recommend their routine use. Experience with investigational therapies is discussed below and holds promise for patients with steroid-refractory disease.
L-HES
Although corticosteroids are also first-line treatment of L-HES, many patients require moderate to high doses (30-60 mg prednisone equivalent daily) to induce and maintain clinical remission. When significant steroid side effects develop or eosinophilia and symptoms persist despite corticosteroid therapy, interferon-α is the preferred second-line agent due to its effects on both eosinophils and T cells. Although in vitro data suggest that interferon-α monotherapy may cause outgrowth of abnormal lymphocyte populations,89 the utility of concomitant low-dose corticosteroid therapy to enhance apoptosis of these cells in patients with L-HES treated with interferon-α is controversial. As in idiopathic HES, other agents, including methotrexate, cyclophosphamide, cyclosporine, and alemtuzumab, have been used as steroid-sparing agents in L-HES with variable success.16,34
Because of an increased risk of T-cell lymphoma, patients with L-HES should be followed closely for clinical and laboratory evidence of neoplastic transformation and the proportion of aberrant T cells (if present) should be assessed by peripheral blood flow cytometry every 6 to 12 months. Additional evaluation, including bone marrow biopsy and imaging studies, should be repeated if there is any suggestion of disease progression. Bone marrow assessment should include cytogenetic analysis, because chromosomal abnormalities, especially 6q deletions, may be an early marker of the development of lymphoma in patients with L-HES.35
Case (continued)
In 2010, 2 years after being diagnosed with idiopathic HES, the patient was enrolled on a clinical trial of mepolizumab. Within 1 week of receiving the first dose (750 mg intravenously), her AEC had normalized. She has remained on mepolizumab (750 mg intravenously every 6 weeks) since that time, with AEC ranging from 0.2 to 0.45 × 109/L. She has required no further corticosteroid therapy, and her cardiac function has improved with resolution of the left ventricular thrombus.
Novel therapies and clinical trials
A number of agents that target eosinophils are currently in clinical development for the treatment of eosinophilic disorders.90,91 Among these, the humanized monoclonal anti-IL-5 antibody, mepolizumab, has been the best studied in HES. After promising results in pilot studies, a double-blind, placebo-controlled trial in 85 PDGFRA-negative patients demonstrated that monthly mepolizumab was safe and effective as a steroid-sparing agent in HES, including L-HES.92,93 Long-term safety and efficacy was confirmed in a second study.92 Mepolizumab is currently available only on clinical protocols for patients with life-threatening HES refractory to standard therapies (as in the case presented) or with EGPA (http://www.clinicaltrials.gov). Additional novel agents currently in trials for the treatment of HES include benralizumab (an afucosylated monoclonal antibody to IL-5 receptor that has shown efficacy in eosinophilic asthma94 ) and dexpramipexole.
Conclusions
HES is a heterogeneous group of disorders with varied etiologies, clinical manifestations, and prognoses. Recent advances in our understanding of the pathogenesis of HES variants combined with the development of less toxic, targeted therapies, such as imatinib and anti-IL-5 antibody, have dramatically improved outcomes in some patients with HES. As the number of therapeutic options continues to expand, the likelihood of response in a given patient and the side effect profile and cost of individual agents will play increasing roles in treatment decisions.
The online version of this article contains a data supplement.
Acknowledgments
The author thanks Drs Paneez Khoury and Peter Weller for their critical review.
This work was funded by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Authorship
Contribution: A.D.K. wrote the paper.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Amy D. Klion, Bldg 4, Room B1-28, 4 Memorial Dr, Bethesda, MD 20892; e-mail: aklion@nih.gov.