In this issue of Blood, Gunasekera et al provide evidence that the high rate of factor VIII (FVIII) inhibitors seen in black hemophilia A (HA) patients is not due to a mismatch between the structure of treatment products and FVIII genotypes common in blacks.1
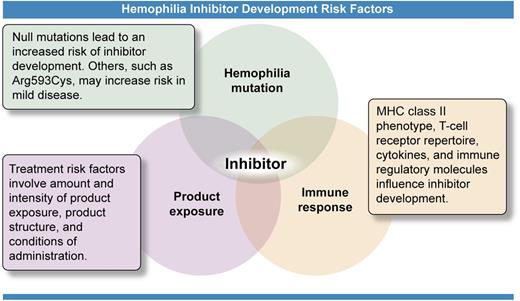
Risk factors for development of inhibitors (neutralizing antibodies) against treatment products used to stop or prevent bleeding in hemophilia patients include 3 major categories. The causative mutation in the gene for FVIII or FIX has been shown to be the most significant risk factor, as whether a gene product is produced or its structure determines how the immune system recognizes the infused protein. The treatment product itself, how much exposure has occurred, and conditions of that exposure provide the trigger for the immune response. Determinants within the immune system control the response through a multiplicity of genes producing a wide phenotypic variability. Interaction of these 3 components, illustrated by the overlapping circles of the Venn diagram, provides each person with hemophilia with a highly individualized risk of developing this complication of treatment at some point during his or her lifetime. Professional illustration by Ken Probst, Xavier Studio.
Risk factors for development of inhibitors (neutralizing antibodies) against treatment products used to stop or prevent bleeding in hemophilia patients include 3 major categories. The causative mutation in the gene for FVIII or FIX has been shown to be the most significant risk factor, as whether a gene product is produced or its structure determines how the immune system recognizes the infused protein. The treatment product itself, how much exposure has occurred, and conditions of that exposure provide the trigger for the immune response. Determinants within the immune system control the response through a multiplicity of genes producing a wide phenotypic variability. Interaction of these 3 components, illustrated by the overlapping circles of the Venn diagram, provides each person with hemophilia with a highly individualized risk of developing this complication of treatment at some point during his or her lifetime. Professional illustration by Ken Probst, Xavier Studio.
Black HA patients have consistently been shown to have an almost twofold higher frequency than white patients of inhibitors, the neutralizing antibodies that can develop against FVIII treatment products and limit their usefulness in stopping or preventing bleeding.2,3 The mismatch hypothesis to explain this disparity was an innovative concept proposed by Viel et al based on their observation that the pattern of 4 nonsynonymous single-nucleotide polymorphisms (ns-SNPs) coding for amino acid changes in normal FVIII structure could be used to define 6 F8 haplotypes, the frequencies of which vary by race.4 The 2 most common haplotypes, H1 and H2, are represented in different full-length recombinant FVIII products used for HA treatment. Among 76 African Americans, 25% had haplotypes other than H1 and H2, and this group had increased odds of having an inhibitor (odds ratio, 3.6; 95% confidence interval, 1.1-12.3; P = .04).4 Subsequent studies of small populations of patients of African ancestry have failed to confirm this finding.5-7 The haplotypes investigated are rare in white populations, and no correlation with inhibitors was found in substantially sized groups of white patients.5,8
Gunasekera et al present the most comprehensive study to date to address this question, using 3 different approaches.1 First, statistical analysis using 174 African American and 198 white HA patients confirmed the increased inhibitor frequency in African Americans but showed no correlation of inhibitor status with ns-SNPs or haplotypes. The only statistically significant finding was a higher inhibitor frequency in patients “potentially exposed to ‘sequence-mismatched’ FVIII” than in those not exposed. As the authors note, this should be interpreted with caution. The exposed group included any patient who had ever received a blood product or plasma-derived factor, including FEIBA. Because FEIBA is used primarily to treat inhibitor patients, its inclusion may bias the results. Second, binding affinities of peptides containing the relevant ns-SNPs to HLA-DRB1 alleles were measured to identify SNP/allele combinations that might increase inhibitor risk. Weak or no binding was observed in 85% of these assays. Among subjects with combinations that did bind, >50% had not developed inhibitors. Binding was far less frequent than predicted by computer algorithms. Third, cultured CD4 T cells from a small number of patients infused with mismatched products were examined by tetramer-guided epitope mapping to determine reactivity with FVIII peptides containing the ns-SNP sequences. Using methods that have successfully demonstrated T-cell epitopes in mild hemophilia patients with high-risk mutations resulting in single amino acid changes, they found no high-avidity binding. The authors conclude that the small number of patients potentially reactive to the neoepitopes presented by mismatched products could not account for the high inhibitor rate seen in African Americans.
If FVIII mismatch is not the answer, where do we go from here? Risk factors for development of inhibitors are complex and interrelated (see figure). The causative gene mutation is the primary determinant of inhibitor risk, controlling whether the gene produces a product, and, if so, how different that product is from the normal protein. More than 2500 unique mutations causing HA have been reported.9 This heterogeneity makes inclusion of mutation in risk factor analysis problematic. African American HA patients have not been found to have differences from white Americans in the type and frequency of mutations,4,5 but mutation type has not been included in all analyses. The use of patient groups with the common intron-22 inversion to control for mutation presents an interesting conundrum. Studies have now shown that the inverted gene does produce 2 products, which include ns-SNPs and remain intracellular; they may result in immune tolerance.10 The uniformity of these products across all intron-22-inversion patients has yet to be demonstrated.
Study of immune response genes is similarly daunting, although it presents perhaps the most likely area for identification of racial differences. Study of 13 331 SNPs in 833 subjects yielded 13 candidate genes for further investigation.11 This large population, however, included only 48 black subjects. Larger numbers of black patients and Hispanics, who also have increased inhibitor risk,2,3 will be required to assess whether their immune risk factors differ from those in whites. Functional studies of the type conducted by Gunasekera et al1 will be necessary to evaluate the validity of any genetic risk factors identified.
Product exposure as a risk factor is an area of intense interest. Inhibitor risk is increased during the first few exposures in patients with severe hemophilia, although patients with mild or moderate disease may have a lifelong risk. Inhibitors may be triggered by intense treatment episodes, inflammation, or other challenges to the immune system during therapy. Product structure differences and product switching remain areas of concern. The problem in risk factor analysis is documenting and quantifying exposure. With US reliance on multiple treatment products, it is difficult to identify patients who have received only a single factor product, other than previously untreated patients, who number <400 affected neonates per year. Assessment of the immunogenicity of individual products or treatment methods requires large numbers. Fewer than 1500 black and 1500 Hispanic patients have been enrolled in surveillance programs in the United States, and recruitment of sufficient numbers for studies has been difficult. Indeed, we have hypothesized that the increased numbers of inhibitors seen may result from attendance at hemophilia treatment centers and study enrollment by only the more severely affected inhibitor patients in these population groups.5
An assessment of all 3 components of risk is required to provide an individual patient with treatment designed to minimize the chance of an inhibitor occurring. Very large studies, perhaps conducted internationally, will be required to quantify these risks and to assess the unique characteristics of population subgroups such as blacks and Hispanics that result in their disproportionate risk.
Conflict-of-interest disclosure: The author declares no competing financial interests.