Key Points
Mutation of the PC-binding domain of EPCR results in viable mice that exhibit procoagulant and proinflammatory phenotype when challenged.
EPCRR84A/R84A mice develop splenomegaly as a result of BM failure, suggesting that EPCR plays an important role in hematopoiesis.
Abstract
The interaction of protein C (PC) with the endothelial PC receptor (EPCR) enhances activated PC (APC) generation. The physiological importance of EPCR has been demonstrated in EPCR knockout mice which show early embryonic lethality due to placental thrombosis. In order to study the role of EPCR independent of PC interaction, we generated an EPCR point mutation knock-in mouse (EPCRR84A/R84A) which lacks the ability to bind PC/APC. EPCRR84A/R84A mice are viable and reproduce normally. In response to thrombotic challenge with factor Xa/phospholipids, EPCRR84A/R84A mice generate more thrombin, less APC, and show increased fibrin deposition in lungs and heart compared with wild-type (WT) mice. EPCRR84A/R84A mice challenged with lipopolysaccharide generate less APC, more interleukin-6, and show increased neutrophil infiltration in the lungs compared with WT controls. Interestingly, EPCRR84A/R84A mice develop splenomegaly as a result of bone marrow (BM) failure. BM transplant experiments suggest a role for EPCR on hematopoietic stem cells and BM stromal cells in modulating hematopoiesis. Taken together, our studies suggest that impaired EPCR/PC-binding interactions not only result in procoagulant and proinflammatory effects, but also impact hematopoiesis.
Introduction
The protein C (PC) pathway plays a major role in inhibiting blood coagulation.1 The pathway is initiated upon binding of thrombin to thrombomodulin (TM) on the surface of vascular endothelial cells. The thrombin-TM complex rapidly converts zymogen PC to its active form activated PC (APC). APC generation is augmented by ∼20-fold in vivo by the endothelial protein C receptor (EPCR), a receptor which binds circulating PC and presents it to the thrombin-TM complex.2 APC, in conjunction with its cofactor protein S, degrades coagulation cofactors Va and VIIIa, thereby attenuating thrombin generation. Congenital or acquired defects in components of the PC pathway are associated with an increased risk of venous thrombosis.3-5
In mice, EPCR gene disruption results in early embryonic lethality due to fibrin deposition and placental thrombosis.6 During embryogenesis, EPCR is expressed primarily on trophoblast giant cells at the fetomaternal boundary.7 Conditional EPCR knockout with specific deletion of EPCR in the developing embryo, but not on trophoblast giant cells, results in viable EPCR-deficient mice (ProcrLox) with no evidence of spontaneous thrombosis.8 EPCR has also been found to be highly expressed on hematopoietic stem cells (HSCs),9 however, the biological role for EPCR on HSCs is unclear because selective deletion of EPCR on hematopoietic cells has minimal effects on systemic PC activation.10
In addition to augmenting PC activation, EPCR also mediates the antiinflammatory, antiapoptotic, and endothelial barrier protective effects of APC.11,12 On endothelial cells, the receptors required for both PC activation (TM and EPCR) and APC-mediated cellular signaling (EPCR and protease activated receptor-1 [PAR-1]) pathways are colocalized in lipid rafts.13 EPCR serves as a coreceptor for APC-mediated cleavage of PAR-1.14 Occupancy of EPCR by PC/APC switches the specificity of PAR-1–dependent signaling from a permeability-enhancing to a barrier-protective response.15 APC-mediated PAR-1 cytoprotective signaling requires β-arrestin recruitment and activation of the disheveled-2 scaffold.16 In vivo, overexpression of EPCR on endothelial cells protects mice against thrombotic or septic challenge17 and inhibition of PC binding to EPCR in baboons converts the response of sublethal concentrations of Escherichia coli into a lethal response.17,18
Currently, there are no animal models to study the biological role of EPCR independent of its interaction with PC/APC. In this study, we generated a point mutation knock-in mouse model harboring a variant of EPCR (R84A) which lacks the ability to bind PC/APC. Our findings suggest that EPCR not only regulates coagulation and inflammation, but also plays an important role in hematopoiesis.
Materials and methods
Generation of WT and R84A mEPCR stable cell lines
Construction and mutagenesis of murine EPCR (mEPCR) was done as previously described.19 Human embryonic kidney cells (HEK293) were transfected with pcDNA3.1(−) vectors containing wild-type (WT) mEPCR or R84A mEPCR complementary DNAs (cDNAs) using Effectene transfection reagent (Qiagen). After 2 weeks of drug selection (400 µg/mL G418), drug-resistant colonies were isolated, and expression of cell surface WT or R84A mEPCR was assessed using a FACSCalibur flow cytometer (Becton Dickinson).
Determination of the affinity of Fl-APC for WT mEPCR and R84A mEPCR
To assess the binding of murine APC to WT or R84A mEPCR, murine APC (kindly provided by Dr Charles Esmon, Oklahoma Medical Research Foundation, Oklahoma City, OK) was labeled at the active site with fluorescein (Fl; Haematological Technologies) as previously described.19 HEK293 cells stably transfected with WT or R84A mEPCR were incubated with increasing concentrations of Fl-APC at 4°C for 15 minutes in the dark. Bound Fl-APC was detected on the fluorescence-1 channel on a FACSCalibur flow cytometer. Values for Kd were determined by fitting binding isotherms with a hyperbolic equation using the TableCurve program (Jandel Scientific).
Generation of homozygous R84A EPCR knock-in mice
All animal care and experimental procedures were approved by the McMaster University Research Ethics Board. In collaboration with GenOway (France), the EPCRR84A/R84A knock-in mouse was generated using the following strategy: the gene-targeting vector consisted of exons 1 to 3, where exon 2 contained the desired R84A point mutation. The targeting vector was introduced into mouse embryonic stem cells of the 129Sv/Pas background and homologous recombinants were selected by polymerase chain reaction (PCR) screening. The EPCR recombined embryonic stem cells were used in blastocyst injections to generate male chimeras. The male chimeras were then bred with female Flp deleter mice to excise the neomycin selection cassette to generate heterozygous mice carrying the R84A knock-in mutation (EPCRWT/R84A). Heterozygous offspring were intercrossed to generate homozygous mice carrying the R84A mutation (EPCRR84A/R84A), and backcrossed 3 generations with C57BL/6 mice.
Hematologic analysis of murine blood
Blood was collected via cardiac puncture into 3.2% sodium citrate and a complete blood count was done using the Hemavet 950FS hematology cell analyzer (Drew Scientific).
Bone marrow transplantation
Recipient mice (10-12 weeks old) were exposed to 14 Gy of γ-irradiation from a 137Cs source using a Gammacel 3000 small animal irradiator. Bone marrow (BM) prepared from the tibias and femurs of donor mice was injected via the retro-orbital sinus. Mice were allowed to recover for 4 weeks before blood was collected and DNA was isolated using the QIAamp DNA Blood Minikit (Qiagen) from blood cells for detection of donor-derived genes by PCR. EPCRR84A/R84ABM/EPCRWT/WT chimeric mice were generated by transplanting BM from EPCRR84A/R84A mice into recipient EPCRWT/WT mice. EPCRWT/WTBM/EPCRR84A/R84A chimeric mice were generated by transplanting BM from EPCRWT/WT mice into recipient EPCRR84A/R84A mice. Control mice were recipient EPCRR84A/R84A mice transplanted with EPCRR84A/R84A BM and recipient EPCRWT/WT mice transplanted with EPCRWT/WT BM. Six months posttransplantation, mice were sacrificed, spleen and BM cells were harvested, and total cell counts were performed using a hemocytometer.
Flow cytometric analysis of spleen and BM cells
Spleen tissue from mice was harvested and made into a single-cell suspension by homogenization using the plunger of a 3-mL syringe. Single-cell suspension of the BM was prepared by flushing the femurs and tibias of WT and EPCRR84A/R84A mice with Dulbecco modified Eagle medium (DMEM). Total cell counts were performed using a hemocytometer. Single-cell suspensions were incubated with FcγRIII/II to block nonspecific binding prior to incubation with the following antibodies: fluorescein isothiocyanate (FITC)-anti-Gr-1 (granulocytes), phycoerythrin (PE)-anti-CD41 (megakaryocytes), PE-Cy7-anti-CD45 (leukocytes), PE-Cy7-anti-B220 (B cells), and allophycocyanin-anti-CD11b (monocyte/macrophage) (BD Biosciences). To identify hematopoietic stem and progenitors cells (HSPCs), spleen, and BM single-cell suspensions were incubated with the following antibodies: allophycocyanin-(anti-CD3, -anti-CD45R, -anti-Gr-1, -anti-CD11b, -anti-TER-119), PE-anti-c-Kit, PE-Cy7-anti-Sca-1 (BD Biosciences). Cells were incubated with antibodies for 30 minutes at 4°C. The stained cells were washed twice and resuspended in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and analyzed using a flow cytometer.
Colony-forming cell assays
Isolated spleen, BM, and peripheral blood cells from mice were added to methylcellulose complete media and plated on 35-mm dishes as described by the manufacturer (R&D Systems). Plated cells were incubated for 8 days in a humidified incubator at 37°C and 5% CO2. Colonies were identified and enumerated using gridded scoring dishes on an inverted light microscope.
Immunoblotting of tissue lysates
Mouse organs (heart, kidney, liver, lung, and spleen) were collected and snap-frozen in liquid N2. Tissue lysates were prepared by incubating tissues in lysis buffer (Millipore), followed by homogenization of tissues. Homogenized tissue was spun down at 5500g for 20 minutes at room temperature and the supernatant was collected. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) of the tissue lysates was performed and protein was transferred onto a nitrocellulose membrane. Immunoblotting was performed using a polyclonal rabbit anti-EPCR antibody (clone G-20; Santa Cruz Biotechnology) and horseradish peroxidase (HRP)-conjugated anti-rabbit antibody (Bio-Rad).
Immunohistochemistry and immunofluorescence
Mouse tissues were collected and snap-frozen in dry ice with optimal cutting temperature embedding medium and stored at −70°C. Tissues were sectioned (8-μm thick) and fixed in ice-cold acetone. Sections were blocked for 1 hour with 5% goat serum. Frozen sections were incubated with a rabbit anti-EPCR polyclonal antibody (clone G-20) overnight at 4°C. Sections were washed with PBS and incubated with a goat anti-rabbit secondary antibody conjugated to Alexa 488 (Life Technologies). To stain nuclear DNA, sections were washed in PBS and incubated with 4,6 diamidino-2-phenylindole (DAPI; Life Technologies).
To collect tissues for fibrin staining of factor Xa (FXa)/phospholipid vesicle (PCPS)-challenged mice, WT and EPCRR84A/R84A mice were exsanguinated by cardiac puncture, and slowly perfused with 20 mL of 0.9% NaCl containing 200 U/mL heparin, followed by fixation of tissues in 10% formalin. Tissues were dissected, paraffin embedded, and processed for sectioning. Tissue sections (5-μm thick) were stained with a polyclonal rabbit anti-human fibrin antibody (Dako). The anti-fibrin antibody was detected by incubation with an alkaline phosphatase goat anti-rabbit secondary antibody, followed by incubation with Vector Red, a substrate that generates a bright-red product (Vector Laboratories). Samples were examined by an Olympus BX41 fluorescent microscope and images collected using an Olympus DP72 camera (Olympus Corporation). For quantification of fibrin, the mean fluorescence intensity (MFI) of 10 images per experimental sample was measured using Slidebook software and corrected for the autofluorescence of the tissue by subtracting the MFI of the negative control. To quantitate the amount of red pulp/white pulp in the spleen of WT and R84A mice, the total spleen area and white pulp area of hematoxylin and eosin (H&E)-stained splenic sections was calculated using ImageJ software. The area of the white pulp and red pulp was then expressed as a percentage of total spleen area.
Thrombotic challenge
To determine the effect of the EPCR R84A mutation on PC activation in vivo, anesthetized EPCRWT/WT, EPCRWT/R84A, and EPCRR84A/R84A mice were given an IV injection (via tail vain) of bovine thrombin (1 nmol/kg; Sigma) or 0.9% NaCl as a control.17 At 8 minutes postinjection, blood samples were collected in 3.2% sodium citrate and 0.01 M benzamidine (Sigma). To determine the effect of EPCR R84A mutation on thrombin generation EPCRWT/WT, EPCRWT/R84A, and EPCRR84A/R84A mice were given an IV injection (via tail vein) of FXa (50 pmol/kg; Haematological Technologies) and phospholipid vesicles (150 nmol/kg; Diapharma) in 0.9% NaCl (supplemented with 0.1% BSA and 2 mM CaCl2) or 0.9% NaCl (supplemented with 0.1% BSA and 2 mM CaCl2) as a control. At 10 minutes postinjection, blood samples were collected. Whole blood was centrifuged at 1500g for 10 minutes at room temperature, plasma collected, and stored at −70°C.
LPS challenge
Lipopolysaccharide (LPS) (1 mg/kg; Sigma) was injected intraperitoneally into EPCRWT/WT and EPCRR84A/R84A mice. Whole blood was collected at 0, 2, 4, 6, and 8 hours post-LPS injection into 3.2% sodium citrate and 0.01 M benzamidine. Plasma was collected for coagulation and cytokine assays. To measure myeloperoxidase (MPO) activity (a measure of neutrophil infiltration), lungs from LPS-challenged mice were collected 24 hours post-LPS challenge and snap-frozen in liquid N2. MPO activity was measured as previously described.20
Analysis of murine PC, APC, TAT, and IL-6 levels
Mouse PC and APC was assayed as described previously.17 Mouse thrombin-antithrombin (TAT) complexes were measured using the Enzygnost TAT kit as described by the manufacturer (Siemens). Mouse interleukin-6 (IL-6) antigen was measured using the Quantikine IL-6 ELISA kit as described by the manufacturer. (R&D Systems)
Statistics
Results are shown as the mean ± the standard error (SE). The Student t test was used to compare values between EPCRWT/WT mice and EPCRR84A/R84A mice. A 1-way analysis of variance (ANOVA) was used to compare values between treatment groups of EPCRWT/WT and EPCRR84A/R84A. A P value < .05 was considered to be statistically significant.
Results
Interaction of murine Fl-APC with WT EPCR and R84A EPCR
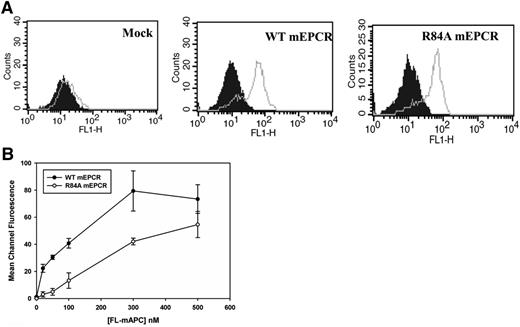
EPCR consists of 2 antiparallel α-helices that sit upon an 8-stranded β-sheet platform.21 Mutagenesis studies of EPCR and the EPCR crystal structure have shown that the PC-binding domain is located at the distal end of the 2 α-helical segments.19,21 To generate a murine EPCR variant which lacks the ability to bind to PC/APC, we mutated Arg84 (a critical residue required for the binding of EPCR to PC/APC) to Alanine. The cDNAs of WT mEPCR and R84A mEPCR were stably transfected into HEK293 cells, and cell surface EPCR expression was confirmed by the binding of FITC-labeled anti-EPCR polyclonal antibody by flow cytometry (Figure 1A). As expected, Fl-mAPC saturably bound WT mEPCR but not R84A mEPCR (Figure 1B). The binding affinity of Fl-mAPC for WT mEPCR is 43 ± 12 nM, consistent with previous reports.19,22 In contrast, R84A mEPCR binding to Fl-mAPC did not saturate even at Fl-mAPC concentrations of 500 nM, which is much higher than the circulating concentration of PC (∼60 nM) (Figure 1B). These studies confirm that the R84A mEPCR variant is expressed on the cell surface but lacks the ability to bind to PC/APC. We are also able to confirm that Fl-mFVIIa and Fl-mFXa does not bind WT or R84A mEPCR (supplemental Figure 1, see supplemental Data available at the Blood Web site).
Flow cytometric analysis of HEK293 cells expressing murine WT or R84A EPCR. (A) HEK293 cells were stably transfected with pcDNA3.1(−) vector only (left), or vectors containing the cDNA encoding murine WT (middle) or R84A (right) EPCR. EPCR expression was measured by incubating transfected HEK293 cells with FITC-IgG isotype control (filled histogram) or FITC-labeled goat polyclonal EPCR antibody (gray line). (B) Stably transfected HEK293 cells expressing WT or R84A mEPCR were incubated with murine Fl-APC (0-500 nM) in the presence of 3 mM CaCl2 and 0.6 mM MgCl2 for 15 minutes at room temperature and binding of Fl-labeled protein to cells expressing mEPCR was analyzed by flow cytometry. IgG, immunoglobulin G.
Flow cytometric analysis of HEK293 cells expressing murine WT or R84A EPCR. (A) HEK293 cells were stably transfected with pcDNA3.1(−) vector only (left), or vectors containing the cDNA encoding murine WT (middle) or R84A (right) EPCR. EPCR expression was measured by incubating transfected HEK293 cells with FITC-IgG isotype control (filled histogram) or FITC-labeled goat polyclonal EPCR antibody (gray line). (B) Stably transfected HEK293 cells expressing WT or R84A mEPCR were incubated with murine Fl-APC (0-500 nM) in the presence of 3 mM CaCl2 and 0.6 mM MgCl2 for 15 minutes at room temperature and binding of Fl-labeled protein to cells expressing mEPCR was analyzed by flow cytometry. IgG, immunoglobulin G.
Generation of EPCRR84A/R84A mice
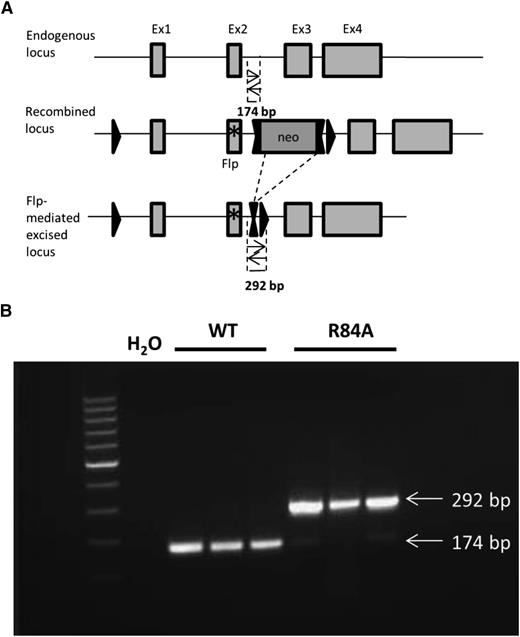
To study the biological role of EPCR in the absence of PC/APC binding in vivo, we generated mice carrying a point mutation in EPCR (R84A). A targeting vector was designed to introduce an R84A point mutation in exon 2 of EPCR, floxed by 2 loxP sites and a neomycin cassette (Figure 2A). Mice homozygous for the EPCR R84A mutation were confirmed by PCR analysis (Figure 2B). Both EPCRR84A/R84A and EPCR WT/R84A mice had normal fertility and lifespans without apparent gross abnormalities.
Detection of mice homozygous for the EPCR R84A mutation by PCR. (A) Schematic diagram of PCR strategy to detect WT or R84A DNA. LoxP sites are represented by single triangles and FRT sites by double triangles. The R84A point mutation in exon 2 is represented by an asterisk (*). PCR amplification of WT genomic DNA results in the detection of a 174-bp fragment, whereas flippase (Flp)-excised genomic DNA yields a 292-bp fragment including an flippase recognition target (FRT) and loxP site. (B) Mouse genomic DNA containing the EPCR Flp-excised allele was tested by PCR. PCR without DNA template (H2O) was used as a negative control.
Detection of mice homozygous for the EPCR R84A mutation by PCR. (A) Schematic diagram of PCR strategy to detect WT or R84A DNA. LoxP sites are represented by single triangles and FRT sites by double triangles. The R84A point mutation in exon 2 is represented by an asterisk (*). PCR amplification of WT genomic DNA results in the detection of a 174-bp fragment, whereas flippase (Flp)-excised genomic DNA yields a 292-bp fragment including an flippase recognition target (FRT) and loxP site. (B) Mouse genomic DNA containing the EPCR Flp-excised allele was tested by PCR. PCR without DNA template (H2O) was used as a negative control.
EPCR expression in organs of WT and EPCRR84A/R84A mice
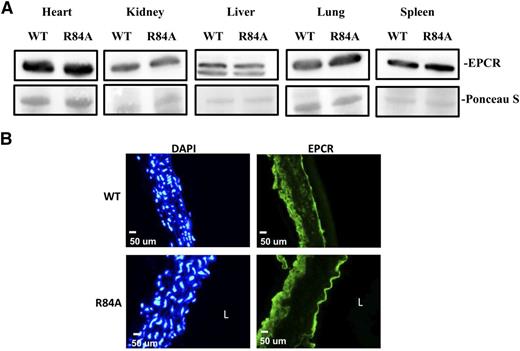
To confirm that the EPCR R84A mutation does not impair the expression of EPCR, we measured EPCR protein from WT and EPCRR84A/R84A tissue lysates by western blot analysis. EPCR protein levels were similar in the heart, kidney, liver, lung, and spleen of WT and EPCRR84A/R84A mice (Figure 3A). Expression of EPCR by aortic endothelial cells was also unaffected by the R84A mutation (Figure 3B).
EPCR expression in EPCRR84A/R84A mice. (A) Western blot analysis of tissue lysates from heart, kidney, liver, lung, and spleen of WT and EPCRR84A/R84A mice. The nitrocellulose membrane was stained with Ponceau S dye to confirm that similar amounts of β-actin protein were loaded in each lane. (B) Frozen sections of aorta from WT and EPCRR84A/R84A mice were fixed with 4% paraformaldehyde and stained with a FITC-labeled rabbit polyclonal antibody specific for EPCR, and DAPI, a nuclear stain used to identify cells. Blots and images are representative of 3 mice per group. L, the lumen.
EPCR expression in EPCRR84A/R84A mice. (A) Western blot analysis of tissue lysates from heart, kidney, liver, lung, and spleen of WT and EPCRR84A/R84A mice. The nitrocellulose membrane was stained with Ponceau S dye to confirm that similar amounts of β-actin protein were loaded in each lane. (B) Frozen sections of aorta from WT and EPCRR84A/R84A mice were fixed with 4% paraformaldehyde and stained with a FITC-labeled rabbit polyclonal antibody specific for EPCR, and DAPI, a nuclear stain used to identify cells. Blots and images are representative of 3 mice per group. L, the lumen.
Baseline levels of PC, APC, and TAT in EPCRWT/WT and EPCRR84A/R84A mice
Next, we determined the baseline levels of PC, APC, TAT, and IL-6 in healthy (ie, unchallenged) EPCRWT/WT and EPCRR84A/R84A mice (Table 1). The plasma PC level was ∼40% higher in EPCRR84A/R84A mice compared with EPCRWT/WT mice, consistent with previous studies which showed that EPCR expression on the endothelium binds PC and decreases the circulating PC level.8,17 Circulating levels of TAT complexes were similar in healthy EPCRR84A/R84A mice compared with EPCRWT/WT (Table 1). APC was not detectable in the EPCRWT/WT or the EPCRR84A/R84A mice. Plasma levels of IL-6 (a proinflammatory cytokine) are higher in EPCRR84A/R84A mice when compared with EPCRWT/WT mice.
Comparison of baseline PC, TAT, and IL-6 antigen levels in WT and EPCRR84A/R84A mice
| Parameter . | WT . | EPCRR84A/R84A . |
|---|---|---|
| PC, μg/mL | 0.9 ± 0.1 | 1.3 ± 0.1* |
| TAT, ng/mL | 5.6 ± 1.0 | 8.4 ± 1.6 |
| IL-6, pg/mL | 15.6 ± 1.5 | 22.0 ± 3.3* |
| Parameter . | WT . | EPCRR84A/R84A . |
|---|---|---|
| PC, μg/mL | 0.9 ± 0.1 | 1.3 ± 0.1* |
| TAT, ng/mL | 5.6 ± 1.0 | 8.4 ± 1.6 |
| IL-6, pg/mL | 15.6 ± 1.5 | 22.0 ± 3.3* |
PC, TAT, and IL-6 antigen levels in healthy WT and EPCRR84A/R84A mice were measured by ELISA. Data represents the mean ± SE; n > 6 for each group.
P < .05.
Thrombotic challenge in EPCRR84A/R84A mice
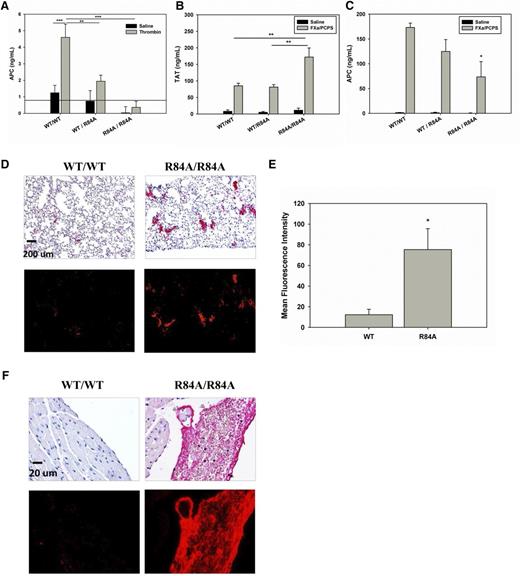
To determine whether mice harboring the EPCR R84A mutation exhibit impaired ability to activate PC, we infused thrombin (1 nmol/kg) into WT, heterozygous, or homozygous mice and measured plasma levels of APC. As shown in Figure 4A, in response to thrombin infusion, plasma levels of APC in EPCR WT/R84A and EPCRR84A/R84A mice were 42% and 8% of those of EPCRWT/WT mice, respectively. These studies confirm that introduction of the R84A mutation in EPCR reduces PC activation in vivo.
Thrombotic challenge of WT EPCRWT/R84A and EPCRR84A/R84A mice. (A) Anesthetized mice were given an IV injection (via tail vein) of bovine thrombin (1 nmol/kg) or 0.9% NaCl. At 8 minutes postinjection, blood samples were collected via the inferior vena cava (IVC) in the presence of 0.01 M benzamidine. APC levels were measured by enzyme capture assay. The solid line indicates the lower limit detection for the assay. (B-C) Anesthetized mice were given an IV injection (via tail vain) of 50 pmol/kg FXa and 75 nmol/kg phospholipid vesicles (PCPS) in the presence of 0.1% BSA and 2 mM CaCl2. At 10 minutes postinjection, blood samples were collected via the IVC. TAT (B) and APC (C) levels were measured by ELISA. (D) Immunohistochemical detection of fibrin deposition in the lungs after FXa/PCPS injection. Red alkaline phosphatase reaction product identifies fibrin under brightfield (top) and fluorescent (bottom) microscopy. (E) Fluorescence quantification shows that the MFI of fibrin staining is greater in the lungs of EPCRR84A/R84A mice when compared with WT mice. (F) Immunohistochemical detection of fibrin deposition in the ventricles of the heart after FXa/PCPS injection. Images are representative of 3 mice per group. Data represents the mean ± SE; *P < .05, **P < .01, ***P < .001; n > 3 per group. ELISA, enzyme-linked immunosorbent assay.
Thrombotic challenge of WT EPCRWT/R84A and EPCRR84A/R84A mice. (A) Anesthetized mice were given an IV injection (via tail vein) of bovine thrombin (1 nmol/kg) or 0.9% NaCl. At 8 minutes postinjection, blood samples were collected via the inferior vena cava (IVC) in the presence of 0.01 M benzamidine. APC levels were measured by enzyme capture assay. The solid line indicates the lower limit detection for the assay. (B-C) Anesthetized mice were given an IV injection (via tail vain) of 50 pmol/kg FXa and 75 nmol/kg phospholipid vesicles (PCPS) in the presence of 0.1% BSA and 2 mM CaCl2. At 10 minutes postinjection, blood samples were collected via the IVC. TAT (B) and APC (C) levels were measured by ELISA. (D) Immunohistochemical detection of fibrin deposition in the lungs after FXa/PCPS injection. Red alkaline phosphatase reaction product identifies fibrin under brightfield (top) and fluorescent (bottom) microscopy. (E) Fluorescence quantification shows that the MFI of fibrin staining is greater in the lungs of EPCRR84A/R84A mice when compared with WT mice. (F) Immunohistochemical detection of fibrin deposition in the ventricles of the heart after FXa/PCPS injection. Images are representative of 3 mice per group. Data represents the mean ± SE; *P < .05, **P < .01, ***P < .001; n > 3 per group. ELISA, enzyme-linked immunosorbent assay.
To test whether mice harboring the R84A EPCR mutation exhibit a more severe procoagulant phenotype when challenged, we infused EPCRWT/WT and EPCRR84A/R84A mice with FXa/PCPS, a procoagulant stimulant. Upon infusion of FXa/PCPS, EPCRR84A/R84A mice had an approximately twofold increase in TAT levels compared with EPCRWT/WT mice whereas the TAT levels were similar between EPCRWT/WT and EPCRWT/R84A mice (Figure 4B). Plasma APC levels were lower in EPCRR84A/R84A compared with EPCRWT/WT or EPCRWT/R84A mice (Figure 4C). Histologic studies show that there is increased fibrin deposition in the lungs of EPCRR84A/R84A mice compared with WT mice (Figure 4D-E). EPCRR84A/R84A mice also showed evidence of large intraventricular fibrin clots in the heart which were absent in EPCRWT/WT mice (Figure 4F).
LPS challenge in EPCRR84A/R84A mice
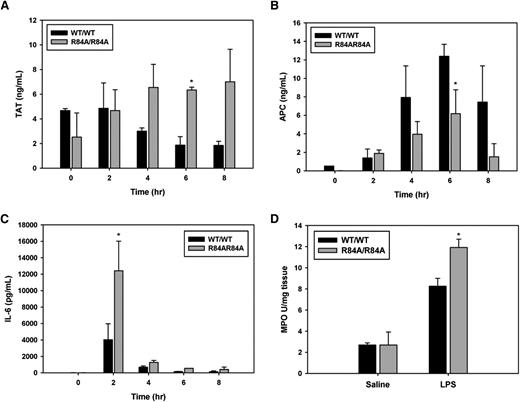
Next, we determined whether mice harboring the R84A EPCR mutation exhibit a more proinflammatory phenotype when challenged with endotoxin. At 6 hours post-LPS injection, EPCRR84A/R84A mice had an approximately threefold increase in TAT levels (Figure 5A), and an approximately twofold decrease in circulating APC (Figure 5B) when compared with EPCRWT/WT mice. In LPS-challenged EPCRR84A/R84A mice, circulating IL-6 was significantly increased at 2 hours post-LPS injection when compared with EPCRWT/WT mice (Figure 5C). Because the lungs are a major site of neutrophil sequestration after LPS challenge, we also measured MPO activity (as a marker of neutrophil infiltration) in the lungs of EPCRWT/WT and EPCRR84A/R84A mice. MPO activity in lung homogenates of EPCRR84A/R84A mice was increased compared with EPCRWT/WT mice 24 hours after LPS injection (Figure 5D).
Inflammatory challenge of WT EPCRWT/R84A and EPCRR84A/R84A mice. Mice were given an intraperitoneal injection of LPS (1 mg/kg). At 0, 2, 4, 6, and 8 hours post-LPS injection; blood samples were collected via the IVC. (A) TAT, (B) APC, and (C) IL-6 levels were measured by ELISA. (D) Lung tissue was collected from mice 24 hours post-LPS injection and snap-frozen in liquid N2 and lung MPO activity was measured as described in “Materials and methods.”
Inflammatory challenge of WT EPCRWT/R84A and EPCRR84A/R84A mice. Mice were given an intraperitoneal injection of LPS (1 mg/kg). At 0, 2, 4, 6, and 8 hours post-LPS injection; blood samples were collected via the IVC. (A) TAT, (B) APC, and (C) IL-6 levels were measured by ELISA. (D) Lung tissue was collected from mice 24 hours post-LPS injection and snap-frozen in liquid N2 and lung MPO activity was measured as described in “Materials and methods.”
EPCRR84A/R84A mice exhibit splenomegaly
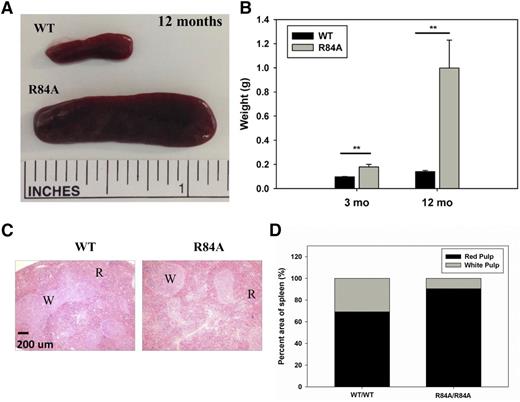
EPCRR84A/R84A mice developed a splenic disorder characterized by splenomegaly (Figure 6A). There was a significant increase in the splenic weight of EPCRR84A/R84A mice compared with WT mice at 3 months of age and further increases in the splenic weight of EPCRR84A/R84A mice at 1 year (Figure 6B). Histologic analysis of the spleen from EPCRR84A/R84A mice revealed an increased amount of red pulp (90.4% ± 0.5%) compared with EPCRWT/WT mice (69.2% ± 3.03%) (Figure 6C-D).
Morphological and histochemical abnormalities in the spleens of EPCRWT/WT and EPCRWT/R84A mice and EPCRR84A/R84A mice. Gross anatomy (A) and weight (in grams) (B) of spleens from EPCRR84A/R84A mice revealed severe splenomegaly compared with WT mice. Data represents the mean ± SE; n > 5 per group. Splenic sections from EPCRR84A/R84A mice stained with H&E at low magnification (×10) (C) reveal an increase in the amount of red pulp (R) but not white pulp (W) compared with splenic sections of age-matched WT mice (D). Images representative of 3 mice per group; **P < .01.
Morphological and histochemical abnormalities in the spleens of EPCRWT/WT and EPCRWT/R84A mice and EPCRR84A/R84A mice. Gross anatomy (A) and weight (in grams) (B) of spleens from EPCRR84A/R84A mice revealed severe splenomegaly compared with WT mice. Data represents the mean ± SE; n > 5 per group. Splenic sections from EPCRR84A/R84A mice stained with H&E at low magnification (×10) (C) reveal an increase in the amount of red pulp (R) but not white pulp (W) compared with splenic sections of age-matched WT mice (D). Images representative of 3 mice per group; **P < .01.
Hematologic analysis of spleen, BM, and peripheral blood in EPCRR84A/R84A mice
Flow cytometric analysis of the spleen and BM was performed to further characterize the cell populations in EPCRR84A/R84A mice (Table 2). In the spleen of EPCRR84A/R84A mice, there was a significant increase in the percentage monocytes/macrophages (CD11b+ cells). The total cell numbers in the BM were decreased in EPCRR84A/R84A mice. Hematologic analysis of peripheral blood revealed circulating platelets were significantly decreased in EPCRR84A/R84A mice compared with EPCRWT/WT mice (Table 3). These studies suggest that EPCRR84A/R84A mice exhibit increased cell numbers in the spleen due to increased extramedullary hematopoiesis as a result of BM failure.
Hematologic analysis of the spleen and BM of EPCRR84A/R84A mice (n > 4 per group)
| . | . | Monocyte/macrophage CD11b+ . | B cells B220+ . | Granulocytes Gr-1+ . | Megakaryocytes CD41+ . | HSPC LSK+ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cells/BW (g) × 105 . | Cells/BW (g) × 105 . | % . | Cells/BW (g) × 105 . | % . | Cells/BW (g) × 105 . | % . | Cells/BW (g) × 105 . | % . | Cells/BW (g) × 105 . | % . | |
| Spleen | |||||||||||
| WT | 28.0 ± 3.46 | 1.61 ± 0.32 | 6.26 ± 1.16 | 18.2 ± 2.19 | 65.2 ± 1.07 | 2.04 ± 0.43 | 7.14 ± 0.75 | 2.83 ± 1.12 | 8.76 ± 2.66 | 0.70 ± 0.33 | 0.85 ± 0.32 |
| R84A | 72.4 ± 14.1 | 9.81 ± 2.45 | 12.5 ± 2.24 | 41.7 ± 9.73 | 55.0 ± 6.78 | 4.96 ± 1.04 | 7.29 ± 1.34 | 8.91 ± 1.26 | 12.5 ± 1.79 | 1.5 ± 1.01 | 1.5 ± 1.12 |
| P | .03 | .02 | .04 | .06 | .2 | .03 | .9 | .02 | .2 | .4 | .5 |
| BM | |||||||||||
| WT | 23.2 ± 1.02 | 11.0 ± 1.40 | 48.3 ± 7.46 | 5.44 ± 0.46 | 22.8 ± 2.32 | 11.4 ± 1.46 | 50.0 ± 7.65 | 4.08 ± 1.05 | 17.1 ± 3.57 | 0.14 ± 0.04 | 0.37 ± 0.11 |
| R84A | 13.2 ± 3.16 | 5.82 ± 1.45 | 53.2 ± 3.33 | 2.19 ± 0.66 | 19.1 ± 0.99 | 5.87 ± 1.67 | 53.1 ± 2.69 | 2.89 ± 0.34 | 22.5 ± 3.8 | 0.15 ± 0.05 | 0.71 ± 0.27 |
| P | .03 | .02 | .54 | .01 | .10 | .04 | .72 | .18 | .36 | .8 | .3 |
| . | . | Monocyte/macrophage CD11b+ . | B cells B220+ . | Granulocytes Gr-1+ . | Megakaryocytes CD41+ . | HSPC LSK+ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cells/BW (g) × 105 . | Cells/BW (g) × 105 . | % . | Cells/BW (g) × 105 . | % . | Cells/BW (g) × 105 . | % . | Cells/BW (g) × 105 . | % . | Cells/BW (g) × 105 . | % . | |
| Spleen | |||||||||||
| WT | 28.0 ± 3.46 | 1.61 ± 0.32 | 6.26 ± 1.16 | 18.2 ± 2.19 | 65.2 ± 1.07 | 2.04 ± 0.43 | 7.14 ± 0.75 | 2.83 ± 1.12 | 8.76 ± 2.66 | 0.70 ± 0.33 | 0.85 ± 0.32 |
| R84A | 72.4 ± 14.1 | 9.81 ± 2.45 | 12.5 ± 2.24 | 41.7 ± 9.73 | 55.0 ± 6.78 | 4.96 ± 1.04 | 7.29 ± 1.34 | 8.91 ± 1.26 | 12.5 ± 1.79 | 1.5 ± 1.01 | 1.5 ± 1.12 |
| P | .03 | .02 | .04 | .06 | .2 | .03 | .9 | .02 | .2 | .4 | .5 |
| BM | |||||||||||
| WT | 23.2 ± 1.02 | 11.0 ± 1.40 | 48.3 ± 7.46 | 5.44 ± 0.46 | 22.8 ± 2.32 | 11.4 ± 1.46 | 50.0 ± 7.65 | 4.08 ± 1.05 | 17.1 ± 3.57 | 0.14 ± 0.04 | 0.37 ± 0.11 |
| R84A | 13.2 ± 3.16 | 5.82 ± 1.45 | 53.2 ± 3.33 | 2.19 ± 0.66 | 19.1 ± 0.99 | 5.87 ± 1.67 | 53.1 ± 2.69 | 2.89 ± 0.34 | 22.5 ± 3.8 | 0.15 ± 0.05 | 0.71 ± 0.27 |
| P | .03 | .02 | .54 | .01 | .10 | .04 | .72 | .18 | .36 | .8 | .3 |
BW, body weight.
Hematologic analysis of EPCRR84A/R84A mice
| Parameter . | WT . | R84A . | Normal range . |
|---|---|---|---|
| WBC, K/μL | 5.1 ± 0.77 | 3.1 ± 0.36 | 1.8-10.7 |
| RBC, M/μL | 7.0 ± 0.27 | 6.0 ± 0.35 | 6.36-9.42 |
| Hematocrit, % | 31.5 ± 0.98 | 32.4 ± 2.0 | 35.1-45.4 |
| Platelets, K/μL | 636 ± 51.9 | 460.5 ± 36.7* | 592-2972 |
| Lymphocytes, K/μL | 4.2 ± 0.64 | 2.4 ± 0.35 | 0.9-9.3 |
| Monocytes, K/μL | 0.08 ± 0.02 | 0.12 ± 0.02 | 0.0-0.4 |
| Neutrophils, K/μL | 0.86 ± 0.15 | 0.59 ± 0.07 | 0.1-2.4 |
| Parameter . | WT . | R84A . | Normal range . |
|---|---|---|---|
| WBC, K/μL | 5.1 ± 0.77 | 3.1 ± 0.36 | 1.8-10.7 |
| RBC, M/μL | 7.0 ± 0.27 | 6.0 ± 0.35 | 6.36-9.42 |
| Hematocrit, % | 31.5 ± 0.98 | 32.4 ± 2.0 | 35.1-45.4 |
| Platelets, K/μL | 636 ± 51.9 | 460.5 ± 36.7* | 592-2972 |
| Lymphocytes, K/μL | 4.2 ± 0.64 | 2.4 ± 0.35 | 0.9-9.3 |
| Monocytes, K/μL | 0.08 ± 0.02 | 0.12 ± 0.02 | 0.0-0.4 |
| Neutrophils, K/μL | 0.86 ± 0.15 | 0.59 ± 0.07 | 0.1-2.4 |
Complete blood count of WT (n = 7) and EPCRR84A/R84A mice (n = 8).
RBC, red blood cell; WBC, white blood cell.
P < .05.
To characterize the progenitor activity of the BM and the spleen in EPCRWT/WT and EPCRR84A/R84A mice, cell colony-forming assays were performed on isolated BM and spleen cells of these mice. There were no observed differences in the number of multipotential progenitors and lineage-restricted progenitors of erythroid, granulocyte, monocyte/macrophage pathways from cultured BM and spleen cell suspensions from EPCRWT/WT and EPCRR84A/R84A mice suggesting HSCs are functional in EPCRR84A/R84A mice (supplemental Figure 2).
BM transplantation
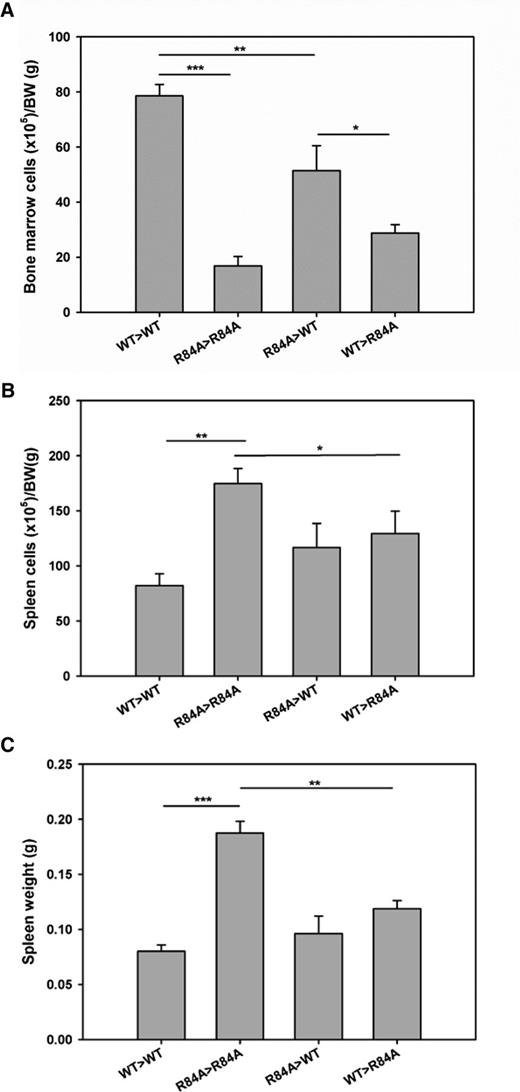
To determine whether BM failure in EPCRR84A/R84A mice is a result of impairment of PC/EPCR binding on hematopoietic cells, we performed BM transplants to generate EPCRR84A/R84ABM/EPCRWT/WT and EPCRWT/WTBM/EPCRR84A/R84A chimeras. Total BM cell counts in EPCRR84A/R84ABM/EPCRWT/WT chimeric mice were significantly decreased compared with control EPCRWT/WTBM/EPCRWT/WT mice (Figure 7A). However, EPCRWT/WTBM was not able to increase the cell count in the BM transplanted EPCRR84A/R84A recipient mice (Figure 7A). As a result, the cellularity of EPCRWT/WTBM/EPCRR84A/R84A chimeric mice was significantly decreased when compared with EPCRR84A/R84ABM/EPCRWT/WT chimeric mice. Despite no increase in total cells in the BM of EPCRWT/WTBM/EPCRR84A/R84A chimeric mice, spleen cell numbers and spleen weight significantly decreased compared with control EPCRR84A/R84ABM/EPCRR84A/R84A mice (Figure 7B-C).
Hematological analysis of the BM and spleen in BM transplanted EPCRWT/WT and EPCRR84A/R84A mice. EPCRR84A/R84ABM/EPCRWT/WT chimeric mice (R84A>WT) were generated by transplanting BM from EPCRR84A/R84A mice into irradiated recipient EPCRWT/WT mice. EPCRWT/WTBM/EPCRR84A/R84A chimeric mice (WT>R84A) were generated by transplanting BM from EPCRWT/WT mice into recipient EPCRR84A/R84A mice. Control mice were recipient EPCRR84A/R84A mice transplanted with EPCRR84A/R84A BM (R84A>R84A) and recipient EPCRWT/WT mice transplanted with EPCRWT/WT BM (WT>WT). Six months post-BM transplantation, mice were sacrificed and total cell counts of BM (A) and spleen (B) were performed. Splenic weight was assessed (C). Data represents the mean ± SE; *P < .05, **P < .01, ***P < .001; n > 5 per group.
Hematological analysis of the BM and spleen in BM transplanted EPCRWT/WT and EPCRR84A/R84A mice. EPCRR84A/R84ABM/EPCRWT/WT chimeric mice (R84A>WT) were generated by transplanting BM from EPCRR84A/R84A mice into irradiated recipient EPCRWT/WT mice. EPCRWT/WTBM/EPCRR84A/R84A chimeric mice (WT>R84A) were generated by transplanting BM from EPCRWT/WT mice into recipient EPCRR84A/R84A mice. Control mice were recipient EPCRR84A/R84A mice transplanted with EPCRR84A/R84A BM (R84A>R84A) and recipient EPCRWT/WT mice transplanted with EPCRWT/WT BM (WT>WT). Six months post-BM transplantation, mice were sacrificed and total cell counts of BM (A) and spleen (B) were performed. Splenic weight was assessed (C). Data represents the mean ± SE; *P < .05, **P < .01, ***P < .001; n > 5 per group.
Discussion
The hemostatic balance is tightly regulated during embryonic development, especially at the fetomaternal interface. During development, maternal fibrinogen serves as a hemostatic agent (to assure that normal bleeding during development of vessels is controlled) and also as an anchor for placental-maternal attachment during embryonic development.23,24 The PC system has been shown to be essential for the maintenance of pregnancy. For example, TM-deficient mice do not survive beyond embryonic day 8.5 (E8.5), and death is caused by tissue factor-initiated coagulation at the fetomaternal interface.25 Deletion of the EPCR gene in mice leads to an increase in fibrin deposition around the giant trophoblast cells at day E9.5 and embryonic lethality before day E10.6 Using conditional knockout strategies, it was shown that EPCR-deficient embryos with EPCR expression on placental giant trophoblast cells can develop normally.8 These studies suggest that impaired PC activation at the fetomaternal interface leads to fibrin deposition and abortion of the embryo. However, we have now demonstrated that mice harboring a variant of EPCR that is deficient in PC binding (Figure 1B) and PC activation in vivo (Figure 4A) can reproduce normally and produce viable offspring.
If impairment in PC activation in the EPCRR84A/R84A mice does not lead to placental thrombosis and embryonic abortion, how is thrombin generation regulated at the fetomaternal interface? It has been demonstrated that EPCR deficiency is not embryonic lethal in genetically modified mice that express low tissue factor (TF) (∼1% of normal), suggesting that decreased thrombin generation rescues EPCR-deficient embryos.8 In this study, we found that the plasma level of PC was ∼40% higher in EPCRR84A/R84 mice compared with WT mice, suggesting that introduction of the R84A mutation into EPCR results in a redistribution of intravascular PC.8,17 We postulate that an increase in plasma PC levels may increase the PC activation rate by the thrombin-TM complex. Although we did not detect measureable differences in plasma APC levels between WT and EPCRR84A/R84A mice (Table 1), it is possible that APC levels in the microcirculation (where the effective concentration of TM on vascular endothelial cells is the highest) are sufficiently high in the EPCRR84A/R84A mice to support embryo development. Alternatively, a role for EPCR, independent of APC may contribute to the viability of EPCRR84A/R84A mice. It has been demonstrated that soluble EPCR (sEPCR), which is present in normal plasma, dose-dependently inhibits FX activation by the sTF/FVIIa complex in the absence of PC/APC.26 During embryonic development, EPCRR84A/R84A may shed enough sEPCR to inhibit FX activation by TF/FVIIa complex, thereby downregulating thrombin generation and preventing placental thrombosis and embryonic lethality in an APC-independent manner.
EPCRR84A/R84A mice have a normal lifespan and showed no evidence of overt pathological thrombosis. However, upon thrombotic or inflammatory challenge EPCRR84A/R84A mice have significant physiological consequences as a result of impaired PC binding to EPCR (Figures 4-5). Similar to EPCR conditional knockout mice (ProcrLox),8 EPCRR84A/R84A mice also have decreased platelet counts (Table 3).8 A decrease in platelet counts and increased extramedullary hematopoiesis may reflect a subacute coagulopathy resulting in hematopoietic compensation and enlargement of the spleen. However, enlargement of the spleen accompanied by a decrease in total cell counts in the BM of EPCRR84A/R84A mice, a phenotype that was not reported in EPCR ProcrLox mice, suggests that EPCR, independent of APC interaction may play an important role in maintaining hematopoietic homeostasis within the BM. Despite decreased cell numbers in the BM, and increased cell numbers in the spleen, percentages of B lymphocytes (B220+ cells), granulocyte progenitors (Gr-1+ cells), megakaryocytes (CD41+), and HSPCs (LSK+) in the BM and spleen were similar between EPCRWT/WT and EPCRR84A/R84A mice (Table 2), and peripheral red and white blood cell counts remained normal in EPCRR84A/R84A mice (Table 3), suggesting that the spleen is able to support normal hematopoiesis.
EPCR is highly expressed on HSCs, and has been used to specifically identify HSCs in murine BM.9 In mice, EPCR expression appears to be limited to HSCs, and is absent on circulating leukocytes.9,27 In support of this, deficiency of EPCR in hematopoietic cells does not impair the anticoagulant and antiinflammatory responses in a mouse model of endotoxemia.10 This is in contrast to humans, in which EPCR expression has been reported in neutrophils, monocytes, eosinophils, and natural killer cells.28-31 EPCR is also present within the BM stroma on endothelial cells, chondrocytes,32 and osteoblasts.33 The results of our BM transplant experiments suggest that EPCR expressed in the stroma may play a greater role in modulating hematopoiesis. In support of this, EPCRWT/WTBM/EPCRR84A/R84A chimeras have a lower BM cellularity than EPCRR84A/R84ABM/EPCRWT/WT chimeras. We postulate that stromal-derived EPCR plays a greater role in modulating hematopoiesis than EPCR expressed by HSCs because the latter makes up only a small percentage of the cell population in the BM. However, our results do not exclude a role for EPCR expressed by HSCs as EPCRR84A/R84ABM/EPCRWT/WT chimeras also had reduced cellularity in the BM (Figure 7A), although not as severe as EPCRWT/WTBM/EPCRR84A/R84A chimeras (Figure 7B-C).
Coagulation regulators including TF, thrombin, TM, and APC have been shown to be present in the small blood vessels of the BM34 and studies have revealed a role for the coagulation system in regulating hematopoiesis. Injection of thrombin into mice can induce rapid mobilization of HSPCs into the peripheral circulation, a process which is dependent on thrombin activation of PAR-1 and secretion of stromal derived factor-1.34,35 However, it is unlikely that increased thrombin activation of PAR-1 in EPCRR84A/R84A as a result of EPCR being unoccupied by PC/APC would result in BM failure, as EPCR ProcrLox mice do not demonstrate a similar phenotype. More recently, a study has demonstrated a role for TM and APC in acceleration of HSPC recovery after irradiation, however, these effects seemed to be independent of APC/EPCR signaling.36 Our results suggest that EPCR in the absence of PC/APC binding may interact with other ligands which may affect hematopoiesis, which would account for the EPCRR84A/R84A phenotype being absent from mice expressing low levels of EPCR. In vitro, EPCR binds Mac-1 directly on monocytes and indirectly on neutrophils via proteinase-3.37,38 EPCR also binds to the γδ T-cell antigen receptor on T cells.39 At this time, it is unclear whether these cellular interactions with EPCR in vivo are relevant to hematopoiesis.
In summary, we have shown that mice harboring a variant of EPCR that lacks the ability to bind to PC/APC exhibit impaired PC activation and increased thrombin generation in response to thrombotic challenge and inflammatory challenge. In addition, we have identified a possible biological role for EPCR in the regulation of hematopoiesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Drs Jeff Weitz and Peter Gross for their helpful comments and suggestions in this project.
This work was supported in part by a grants-in-aid from the Heart and Stroke Foundation (NA6311 and 00050) and the Canadian Institutes for Health Research (grant MOP-106503). P.Y. is the recipient of a graduate scholarship from the Chinese Scholarship Council.
Authorship
Contribution: L.P., P.Y., and D.J.D. performed the experiments; B.L.T. designed experiments; and L.P. and P.C.L. designed the research study, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Patricia C. Liaw, Thrombosis and Atherosclerosis Research Institute, Hamilton General Hospital Campus, 237 Barton St East, Hamilton, ON, L8L 2X2, Canada; e-mail: patricia.liaw@taari.ca.