Key Points
Kindlin-3–β2-integrin signaling in neutrophils is involved in regulation of both neutrophil recruitment and NET release.
Disrupting the crosstalk between kindlin-3 and β2-integrins in neutrophils with a blocking peptide preferentially attenuates NET release.
Abstract
Kindlin-3 essentially supports integrin activation in blood cells. Absence of kindlin-3 in humans causes leukocyte adhesion deficiency-III characterized with severe bleeding disorder and recurrent infections. Previously, we generated kindlin-3 knock-in (K3KI) mice carrying an integrin-interaction disrupting mutation in kindlin-3 and verified the functional significance of the binding of kindlin-3 to integrin αIIbβ3 in platelets. Here, using K3KI mice, we functionally evaluate the crosstalk between kindlin-3 and β2-integrins in neutrophils. Although the kindlin-3 mutant in K3KI neutrophils is normally expressed, its binding ability to β2-integrins in neutrophils is disabled. In vitro and in vivo analyses disclose that β2-integrin–mediated K3KI neutrophil adhesion and recruitment are significantly suppressed. Interestingly, the ability of releasing neutrophil extracellular traps (NETs) from K3KI neutrophils is also compromised. Substantially, a peptide derived from the integrin β2 cytoplasmic tail that can inhibit the interaction between kindlin-3 and β2-inegrins significantly jeopardizes NET release without affecting neutrophil adhesion and recruitment under the experimental conditions. These findings suggest that crosstalk between kindlin-3 and β2-integrins in neutrophils is required for supporting both neutrophil recruitment and NET release, but the involved regulatory mechanisms in these two cellular events might be differential, thus providing a novel therapeutic concept to treat innate immune-related diseases.
Introduction
Neutrophil recruitment and neutrophil extracellular trap (NET) release are hallmarks of innate immune responses. The engagement of β2-integrins on neutrophils with their ligands mediates firm adhesion of neutrophils to inflamed endothelium1,2 and may also be involved in regulating NET release.3,4 Ligand-binding capability of β2-integrins on leukocytes is tightly regulated by a process of integrin activation, which is triggered by extracellular agonists and eventually implemented by the integrin α/β cytoplasmic tail (CT) binding proteins.5,6 Kindlins are recently identified integrin β CT binding proteins, and kindlin-3, one of the 3 kindlin family members, is mainly expressed in hematopoietic cells.7-9 Kindlin-3 mutants in humans are responsible for type III leukocyte adhesion deficiency (LAD-III) with severe bleeding tendency and recurrent infections due to the dysfunction of integrins in both leukocytes and platelets.10,11 Since the first case was described in 1997, a list of LAD-III patients from more than 20 families have been reported so far. The identified kindlin-3 mutations in these patients vary but all result in undetectable expression of kindlin-3 protein in blood cells.12,13 Because kindlin-3 can interact with multiple signaling molecules in addition to integrins,14,15 and knockout of kindlin-3 can alter the expression of multiple molecules in blood cells,16 it is obvious that the functional consequence of the crosstalk between kindlin-3 and β2-integrins in neutrophils could not be simply concluded from the studies of either LAD-III patients or kindlin-3 knockout mice,17 and needs to be further delineated with more targeted methods. Here, using the established kindlin-3 knock-in (K3KI) mice,18 we pinpoint the differential involvement of the interaction between kindlin-3 and β2-integrins in regulating neutrophil recruitment and NET release, and establish a novel strategy for selectively restricting NET release in innate immune response.
Study design
Mice
K3KI mice that carry an integrin-interaction disrupting mutation in kindlin-3 were generated as previously described.18 Inbred wild-type (WT) littermates were used as controls to confront comparative studies. All the mice used in this study were 8 to 12 weeks of age. All the animal studies included in this work have been approved by Institutional Animal Care and Use Committee.
Analyses of neutrophil adhesion and recruitment in vitro and in vivo
Bone marrow (BM) neutrophils were isolated from mice using a discontinuous Percoll gradient method as described in the literature.19 Viability and purity of the isolated neutrophils were verified by trypan blue and Gr1 staining. The expression of cell surface β2-integrin subunits and intracellular kindlin-3 protein were quantified by flow cytometry and western blotting, respectively. Neutrophil adhesion and spreading mediated by β2-integrins were evaluated in vitro on immobilized fibrinogen, intercellular adhesion molecule-1, or tumor necrosis factor-α primed endothelial cells. Neutrophil recruitment in innate immune responses was evaluated in vivo using 2 established mouse models. (1) Endotoxemia: mice were intraperitoneally injected with lipopolysaccharide (LPS) (1 mg/kg). The livers of endotoxemic mice were perfused and collected 4 hours after LPS administration and used for measuring myeloperoxidase (MPO) activity and histologic studies. (2) Deep vein thrombosis (DVT): mice were anesthetized by isoflurane inhalation and survival surgery was performed to ligate the portion of the inferior vena cava (IVC) below the renal veins. The associated side branches were either ligated or cauterized. The IVC tissues were collected 2 or 6 hours later after the ligation and used for measuring the formed thrombi and histologic studies.
Quantification of NET release in vitro and in vivo
For quantifying NET release in vitro, BM neutrophils were allowed to reside on chamber slides precoated with poly-l-lysine and stimulated with the indicated agonists, followed by fixation, permeabilization, and fluorescence staining with Hoechst for DNA. Alternatively, the stimulated BM neutrophils were incubated with micrococcal nuclease (MNase) for 10 minutes at room temperature to detach the released NETs from neutrophils, and MNase activity was inactivated by adding EDTA (5 mM) before collecting the supernatants. For evaluating NET release in vivo, blood samples were collected from mice before and after endotoxemic induction. The soluble NETs in the collected supernatants and blood plasma samples were quantified using SYTOX green assays (Invitrogen) and verified by DNA-histone and DNA-MPO enzyme-linked immunosorbent assays as previously described.4,20
Results and discussion
Interaction of kindlin-3 and β2-integrins essentially supports neutrophil adhesion and recruitment
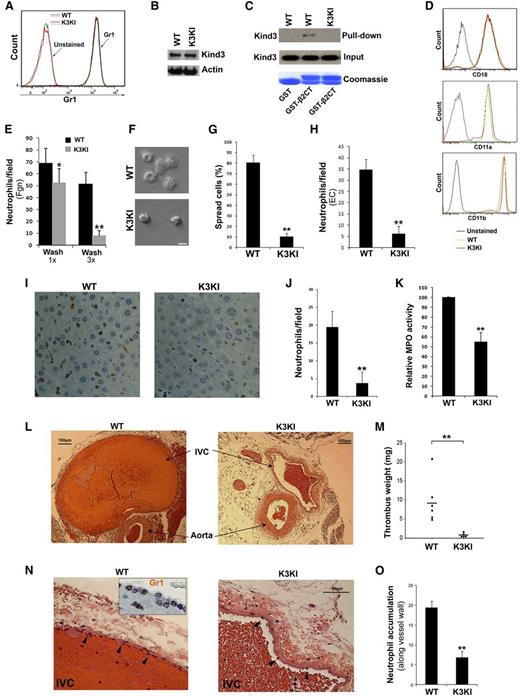
BM neutrophils of mice that were stained positively with Gr1 were used for in vitro studies (Figure 1A). The kindlin-3 mutant in K3KI mice that carries a specific integrin-interaction disrupting substitution (Q597W598/AA) was expressed normally in neutrophils (Figure 1B), but it failed to interact with the integrin β2 CT as compared with the WT form (Figure 1C). In contrast to kindlin-3–deficient neutrophils that show altered expression of integrin subunits,17 cell surface expression of the β2-integrin subunits, αM, αL, and β2 on K3KI neutrophils were unchanged (Figure 1D). These results suggest that K3KI mice provide a valid means to define the functional significance of the direct contact between kindlin-3 and β2-integrins in neutrophils. Adhesion and spreading of the phorbol myristate acetate (PMA)-stimulated K3KI neutrophils on immobilized fibrinogen were significantly suppressed (Figure 1E-G). Similar results were also observed on immobilized intercellular adhesion molecule-1 (not shown). Interestingly, initial adhesion still could occur substantially (Figure 1E, with 1× wash), indicating that kindlin-3 is more essential for supporting β2-integrin–mediated neutrophil firm adhesion, consistent with the previous findings.21 In addition, K3KI neutrophils exhibited significantly compromised adhesion to inflamed endothelial cells (Figure 1H), suggestive of the functional importance of direct interaction between kindlin-3 and integrins in neutrophils. Further, we evaluated neutrophil recruitment in innate immune responses in mice. First, we found that neutrophil recruitment in the sinusoids of endotoxemic livers in K3KI mice was significantly suppressed, as evaluated by immunohistochemical (IHC) staining of Gr1 and MPO activity in the liver tissues (Figure 1I-K).22 Second, a complete IVC ligation model in mice was employed to induce DVT formation, in which it has been demonstrated that the initial neutrophil recruitment is critically involved.23 The IVC anatomy was examined in both K3KI and WT mice and no differences were identified between them. After ligation of the IVC for 2 hours, significant neutrophil recruitment could be observed in the IVC of WT mice even without the presence of obvious thrombi, but this did not occur in K3KI mice (data not shown). After ligation of the IVC for 6 hours, thrombus was well formed in WT mice but substantially inhibited in K3KI mice (Figure 1L-M); in addition, neurophil accumulation along the IVC vessel walls in K3KI mice was significantly less than that in WT mice (Figure 1N-O). It is worth pointing out that the compromised neutrophil recruitment in K3KI mice was not due to the neutrophil counts in peripheral blood because K3KI mice actually displayed significant granulocytosis (Figure 2B). Preliminarily, we also observed that adoptive transfer of WT neutrophils could partially restore thrombus formation in the ligated IVC in K3KI mice, verifying the important role of neutrophils in promoting DVT formation. Since the kindlin-3 mutant in K3KI mice was specifically designed for disrupting integrin recognition with limited effects, if there are any, on other binding partners, the above findings demonstrate for the first time that the direct interaction of kindlin-3 and integrins is functionally required for supporting neutrophil recruitment in acute immune challenges.
Adhesion and recruitment of K3KI neutrophils are suppressed in vitro and in vivo. (A) BM neutrophils isolated from WT and K3KI mice were stained with an anti-Gr1 antibody and analyzed by flow cytometry. (B) Western blotting to measure kindlin-3 expression in BM neutrophils. (C) Pull-down assay to measure interaction of glutathione S-transferase (GST)-β2CT protein with kindlin-3 in neutrophil lysates. GST alone was used as a control. Bound kindlin-3 protein in the precipitates was evaluated by western blotting; GST and GST-β2CT proteins were evaluated by Coomassie blue staining. (D) Surface expression of the β2-integrin subunits on BM neutrophils of WT and K3KI mice were measured by flow cytometry. (E-G) BM neutrophils were incubated with immobilized Fgn for 30 minutes in the presence of PMA (20 nM). The adherent cells were fixed and counted by microscopy (E); the representative images were exhibited (F); and percentage of spread cells were analyzed (G). Scale bar = 10 µm. (H) PMA-stimulated BM neutrophils were allowed to adhere to tumor necrosis factor-α primed EC monolayer, and the adherent neutrophils were fixed, stained with an fluorescein isothiocyanate-conjugated anti-Gr1 antibody and quantified under a fluorescence microscope. (I) Representative histologic images (×20 objective) of liver tissues isolated from the endotoxemic mice with IHC staining of Gr1. (J) Gr1-positive neutrophils recruited in the livers of endotoxemic mice. (K) MPO was extracted from the fresh liver tissues of endotoxemic mice and MPO activity was measured as described before.22 (L) Representative histologic images (×4 objective) of the ligated mouse IVC tissues with staining of hematoxylin and eosin. (M) Thrombi formed in the ligated IVC tissues were isolated and quantified by weighing. (N) Neutrophil recruitment was visualized in histologic sections (×20 objective) of the ligated IVC tissues with staining of hematoxylin and eosin. IHC staining for Gr1 was shown in the insertion (×40 objective). (O) The accumulated neutrophils along the vessel walls (arrowhead) in the ligated IVC tissues were quantified. Data represent mean ± SEM of 3 or more independent experiments; *P < .05; **P < .01 (paired Student t test). EC, endothelial cell; Fgn, fibrinogen.
Adhesion and recruitment of K3KI neutrophils are suppressed in vitro and in vivo. (A) BM neutrophils isolated from WT and K3KI mice were stained with an anti-Gr1 antibody and analyzed by flow cytometry. (B) Western blotting to measure kindlin-3 expression in BM neutrophils. (C) Pull-down assay to measure interaction of glutathione S-transferase (GST)-β2CT protein with kindlin-3 in neutrophil lysates. GST alone was used as a control. Bound kindlin-3 protein in the precipitates was evaluated by western blotting; GST and GST-β2CT proteins were evaluated by Coomassie blue staining. (D) Surface expression of the β2-integrin subunits on BM neutrophils of WT and K3KI mice were measured by flow cytometry. (E-G) BM neutrophils were incubated with immobilized Fgn for 30 minutes in the presence of PMA (20 nM). The adherent cells were fixed and counted by microscopy (E); the representative images were exhibited (F); and percentage of spread cells were analyzed (G). Scale bar = 10 µm. (H) PMA-stimulated BM neutrophils were allowed to adhere to tumor necrosis factor-α primed EC monolayer, and the adherent neutrophils were fixed, stained with an fluorescein isothiocyanate-conjugated anti-Gr1 antibody and quantified under a fluorescence microscope. (I) Representative histologic images (×20 objective) of liver tissues isolated from the endotoxemic mice with IHC staining of Gr1. (J) Gr1-positive neutrophils recruited in the livers of endotoxemic mice. (K) MPO was extracted from the fresh liver tissues of endotoxemic mice and MPO activity was measured as described before.22 (L) Representative histologic images (×4 objective) of the ligated mouse IVC tissues with staining of hematoxylin and eosin. (M) Thrombi formed in the ligated IVC tissues were isolated and quantified by weighing. (N) Neutrophil recruitment was visualized in histologic sections (×20 objective) of the ligated IVC tissues with staining of hematoxylin and eosin. IHC staining for Gr1 was shown in the insertion (×40 objective). (O) The accumulated neutrophils along the vessel walls (arrowhead) in the ligated IVC tissues were quantified. Data represent mean ± SEM of 3 or more independent experiments; *P < .05; **P < .01 (paired Student t test). EC, endothelial cell; Fgn, fibrinogen.
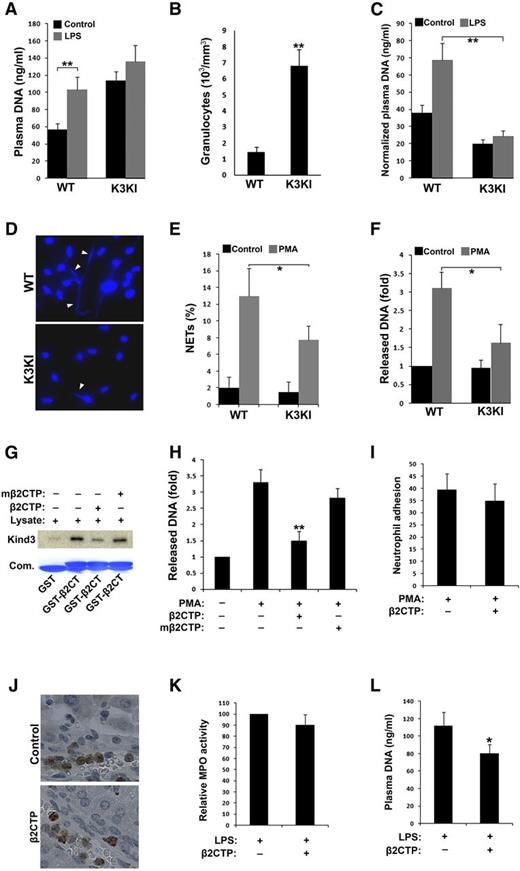
Crosstalk between kindlin-3 and β2-integrins modulates NET release in vitro and in vivo. (A) Blood samples (∼100 µl/mouse) were collected from mice before and after LPS treatment and mixed with the same volume of phosphate-buffered saline. Diluted plasma was obtained by centrifugation and plasma DNA was measured by SYTOX green. (B) Counts of granulocytes in peripheral blood of mice. (C) Plasma DNA were normalized by the granulocyte counts in peripheral blood (per million). (D) BM neutrophils were treated with PMA (20 nM) for 2 hours followed by fixation, permeabilizaion, and labeling nuclear DNA with Hoechst to display released NETs (arrowheads). (E) Quantification of formed NETs from (D). (F) NETs were solubilized from PMA-stimulated neutrophils by MNase and then quantified by SYTOX green. (G) Interaction of GST-β2CT with kindlin-3 was evaluated by pull-down assays in the presence of the peptide derived from the integrin β2 CT (β2CTP: GRKKRRQRRRFKSATTTVMNPKFAES) or its mutated one (mβ2CTP: GRKKRRQRRRFKSAAAAVMNPKAAES) at a concentration of 20 µM. (H) BM neutrophils of WT mice were pretreated with the membrane-permeable β2CTP or mβ2CTP (20 µM) and NET release was measured as described in (F). (I) BM neutrophils of WT mice were pretreated with the β2CTP (20 µM), and their adhesive ability to fibrinogen was quantified as described in Figure 1E. (J-L) WT mice were administrated with the β2CTP (5 mg/kg) by IV injection before LPS treatment to induce endotoxemia. The livers were harvested from the endotoxemic mice for IHC staining of Gr1 (J) and measuring MPO activity (K); meanwhile, blood samples were collected for quantifying plasma DNA (L). Data represent mean ± SEM of 3 or more independent experiments; *P < .05; **P < .01 (paired Student t test).
Crosstalk between kindlin-3 and β2-integrins modulates NET release in vitro and in vivo. (A) Blood samples (∼100 µl/mouse) were collected from mice before and after LPS treatment and mixed with the same volume of phosphate-buffered saline. Diluted plasma was obtained by centrifugation and plasma DNA was measured by SYTOX green. (B) Counts of granulocytes in peripheral blood of mice. (C) Plasma DNA were normalized by the granulocyte counts in peripheral blood (per million). (D) BM neutrophils were treated with PMA (20 nM) for 2 hours followed by fixation, permeabilizaion, and labeling nuclear DNA with Hoechst to display released NETs (arrowheads). (E) Quantification of formed NETs from (D). (F) NETs were solubilized from PMA-stimulated neutrophils by MNase and then quantified by SYTOX green. (G) Interaction of GST-β2CT with kindlin-3 was evaluated by pull-down assays in the presence of the peptide derived from the integrin β2 CT (β2CTP: GRKKRRQRRRFKSATTTVMNPKFAES) or its mutated one (mβ2CTP: GRKKRRQRRRFKSAAAAVMNPKAAES) at a concentration of 20 µM. (H) BM neutrophils of WT mice were pretreated with the membrane-permeable β2CTP or mβ2CTP (20 µM) and NET release was measured as described in (F). (I) BM neutrophils of WT mice were pretreated with the β2CTP (20 µM), and their adhesive ability to fibrinogen was quantified as described in Figure 1E. (J-L) WT mice were administrated with the β2CTP (5 mg/kg) by IV injection before LPS treatment to induce endotoxemia. The livers were harvested from the endotoxemic mice for IHC staining of Gr1 (J) and measuring MPO activity (K); meanwhile, blood samples were collected for quantifying plasma DNA (L). Data represent mean ± SEM of 3 or more independent experiments; *P < .05; **P < .01 (paired Student t test).
Association of kindlin-3 with β2-integrins in neutrophils is involved in regulation of NET release
NETs are composed of chromatin fibers and multiple granule proteins such as MPO that are released from activated neutrophils. NET release is one of the important strategies employed by neutrophils in innate immune defense.24 Because β2-integrins may possibly participate in regulating NET release,3,4 we next explored the functional relevance of kindlin-3/integrin interactions and NET release in K3KI mice. Due to the observations that neutrophil recruitment in tissues could be significantly reduced in K3KI mice during innate immune responses (Figure 1I-K, N-O), quantification of tissue NETs may not be suitable for confronting comparative studies between K3KI and WT mice. Therefore, we instead measured plasma DNA in mice for evaluating the overall changes. In WT mice, endotoxemic conditions led to a significant increase of the soluble plasma DNA as measured by SYTOX green (Figure 2A). Similar results were obtained when performing the measurements with DNA-MPO or DNA-histone enzyme-linked immunosorbent assay (data not shown), suggestive of a possibly enhanced NET release in the blood. However, plasma DNA in endotoxemic K3KI mice was only slightly altered (Figure 2A). Interestingly, the basal level of plasma DNA in K3KI mice was significantly higher than that in WT mice, which could be ascribed to granulocytosis in K3KI mice (Figure 2B). Practically, after normalization with the granulocyte counts in peripheral blood, the plasma DNA level in K3KI mice was significantly lower than that in WT mice under both basal and endotoxemic conditions (Figure 2C), suggesting that K3KI neutrophils may display a compromised function of releasing NETs. To verify this possibility, the capacity of NET release from the isolated BM neutrophils was tested in vitro, and we found that both the numbers of DNA fibers attached to and the amount of released DNA from the stimulated K3KI neutrophils were less than those of the WT counterparts (Figure 2D-F). Taken together, these results demonstrate that the interaction of kindlin-3 with integrins in neutrophils may be involved in regulating NET release.
Neutrophil recruitment and NET release are differentially regulated by the crosstalk between kindlin-3 and β2-integrins in innate immune response
To further explore the therapeutic potential of integrin–kindlin-3 signaling in neutrophils, we employed a specific peptide that comprises both the kindlin-binding site from the integrin β2 CT and the membrane-penetrating transactivator of transcription peptide (termed as β2CTP). As expected, the β2CTP but not its mutant (mβ2CTP) that is unable to bind to kindlin-317,25 significantly inhibited kindlin-3 binding to the integrin β2 CT (Figure 2G). Importantly, the β2CTP (20 µM) suppressed DNA release from neutrophils in vitro but the mβ2CTP did not (Figure 2H). In contrast, the β2CTP at the same concentration failed to inhibit β2-integrin–mediated neutrophil adhesion (Figure 2I). However, when a higher concentration of β2CTP (100 µM) was used, we could observe that both DNA release and neutrophil adhesion were able to be inhibited (data not shown). These results demonstrate that partial disruption of the crosstalk between kindlin-3 and integrins is capable of attenuating NET release but may not be sufficient to inhibit neutrophil adhesion, indicating that a strategy of inefficient blockage of integrin–kindlin-3 signaling could be useful for selectively constraining NET release without adversely affecting neutrophil adhesion. In fact, the amount of released NETs in plasma was significantly reduced, whereas neutrophil recruitment to livers was unchanged in endotoxemic mice treated with the β2CTP (Figure 2J-L). The differential dependence of neutrophil recruitment and NET release on the crosstalk between kindlin-3 and integrins indicates that kindlin-3 may regulate these two cellular events by distinct mechanisms. For example, blockage of kindlin-3/integrin interactions in either K3KI or β2CTP-treated neutrophils could increase the availability of kindlin-3 in cells for some integrin-independent activities, and this might be possibly related to the regulation of NET release. Although further study is required to delineate the mechanism, the results from this study highlight that partial restriction of the crosstalk between kindlin-3 and integrins in neutrophils may be an effective strategy for controlling NET release in innate immunity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ela Pluskota and Ed Plow (Cleveland Clinic) for providing technical support.
This work was supported by grants from the Blood Center Research Foundation of the BloodCenter of Wisconsin, the National Natural Science Foundation of China (81270579 and 31370748), and the Medical College of Wisconsin Cancer Center/Wisconsin Breast Cancer Showhouse.
Authorship
Contribution: Z.X., J.C., and J.G. contributed to acquisition of the data and reviewed the manuscript; G.C.W. and F.C. contributed to interpretation of the data and reviewed the manuscript; Y.-Q.M. contributed to design of the experiments, analysis of the data, and wrote the manuscript; and all authors approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yan-Qing Ma, Blood Research Institute, BloodCenter of Wisconsin, 8727 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: yanqing.ma@bcw.edu.
References
Author notes
Z.X. and J.C. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal