Key Points
Children treated for ALL without cranial radiation display significant IQ deficits of 6 to 8 points compared with healthy controls.
Moderate deficits occur in other neurocognitive domains including working memory, information processing speed, and fine motor functioning.
Abstract
Therapy for childhood acute lymphoblastic leukemia (ALL) is associated with 5-year survival rates of ∼90% even after largely eliminating cranial radiation. This meta-analysis assesses the long-term neurocognitive functioning after chemotherapy-only regimens among survivors of childhood ALL. We conducted a systematic review to identify studies that evaluated long-term neurocognitive functioning following treatment of ALL by searching MEDLINE/PubMed, Database of Abstracts of Reviews of Effects, and secondary sources. Studies were included if ALL survivors were in continuous first remission, did not receive any radiation, were at least ≥2 years off therapy or ≥5 years since diagnosis, and were compared with a healthy control group. Weighted mean differences with 95% confidence intervals (CIs) were calculated. Ten nonexperimental studies met all eligibility criteria and included 509 patients and 555 controls. Meta-analysis demonstrated statistically significant moderate impairment across multiple neurocognitive domains evaluated, with intelligence most affected. Significant differences in standard deviation (SD) scores were found for Full Scale intelligence quotient (IQ) (−0.52 SD; 95% CI, −0.68 to −0.37), Verbal IQ (−0.54 SD; 95% CI, −0.69 to −0.40), and Performance IQ (−0.41 SD; 95% CI, −0.56 to −0.27); these SD scores correspond to changes in IQ of 6 to 8 points. Working memory, information processing speed, and fine motor domains were moderately, but statistically significantly, impaired. Meta-analysis of ALL survivors treated without cranial radiation demonstrated significant impairment in IQ and other neurocognitive domains. Patients and their families should be informed about these potential negative effects to encourage surveillance and educational planning. Both preventive and intervention strategies are needed.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common malignancy in children, accounting for 18% of all cancers and 74% of all leukemias.1 As a result of improvements in treatment and supportive care over recent decades, the current 5-year relative survival rate is 89% for children diagnosed with ALL before age 20 years1 and exceeds 93% for children with standard risk features diagnosed before age 10 years.2 Because the vast majority of children with ALL are expected to survive their disease, it is important to understand the long-term burden of therapy.
Neurocognitive and psychological difficulties as a result of chemotherapy have been documented in survivors of adult cancer across a variety of cancer types.3 However, most pediatric studies have focused on children with brain tumors or central nervous system (CNS)-directed radiation treatment.4 Although the deleterious neurocognitive effects of cranial radiation in ALL patients have been well-documented,5-10 upfront treatment of ALL now rarely involves cranial radiation and instead accomplishes CNS prophylaxis with systemic and intrathecal chemotherapy.11,12 Results have varied on the negative effects of chemotherapy without radiation in pediatric cancer populations.13-15 Individual studies in the past have shown some negative effects of treatment. However, most of these studies were hindered by small sample size and lack of appropriate controls, which makes it challenging to interpret and generalize their findings.11-14 Conclusions from systematic reviews have been mixed,16-18 largely because of reliance on qualitative methods to assess outcomes18 and inclusion of studies with patients who had very recently completed therapy or without a control population.16-18 Because neurocognitive impairment takes time to develop and/or be detected, early assessments are likely not adequate. Furthermore, because many of these neurocognitive measures are correlated with demographic factors, such as socioeconomic status, comparison with an appropriate healthy control group is particularly important.19
We therefore conducted a systematic review and meta-analysis to objectively and quantitatively evaluate the existing literature using more stringent criteria to overcome some of the limitations of earlier reviews. The aim was to determine the pattern and severity of long-term neurocognitive functioning impairments following chemotherapy-only treatment among survivors of childhood ALL.
Materials and methods
Search strategy
Our search and evaluation of articles were conducted in accordance with the guidelines set forth by the Preferred Reporting Items of Systematic Reviews and Meta-Analysis statement.20 Electronic searches of the MEDLINE/PubMed (1966 to 1 September 2014) and the Database of Abstracts of Reviews of Effects (1994 to 1 September 2014) were performed combining disease-specific terms and outcome-specific terms using the following search algorithm: ((acute lymphocytic leukemia) OR (acute lymphoblastic leukemia) OR (childhood leukemia) OR (pediatric leukemia) OR (ALL) OR (leukemia)) AND ((neuropsychological) OR (neurocognitive) OR (cognitive) OR (memory) OR (intelligence) OR (attention) OR (processing speed) OR (IQ) OR (intelligence quotient) OR (achievement) OR (math) OR (reading) OR (motor functioning)). There was no language limitation. This literature search was supplemented by reviewing relevant citations in the initial studies identified, and previous review articles examining similar outcomes.15-18
Inclusion and exclusion criteria
Studies included in this analysis evaluated neurocognitive outcomes in ALL survivors who met the following eligibility criteria: (1) age <21 years at ALL diagnosis, (2) ≥5 years postdiagnosis or ≥2 years off treatment in continuous first remission, (3) no history of cranial irradiation (focally or as part of total body irradiation), (4) cancer-free at the time of neurocognitive assessment, and (5) comparison with a healthy control group. The healthy comparison group was required in order to evaluate the data using traditional meta-analysis methods. The ALL populations were diverse, and appropriate race- and socioeconomic-specific normative comparative scores were not available across all domains. There were no major inclusion criteria for the healthy controls, except that they came from the surrounding community or geographic area. For studies that included multiple patient groups who received different treatment regimens, only the subsample that received a chemotherapy-only treatment of ALL was included in the analyses.
All included studies were quasi-experimental, also known as nonrandomized intervention studies. These types of studies aim to demonstrate causality between an intervention and an outcome without using randomization.21,22 Because ALL therapy requires chemotherapy for cure and no healthy child would ever be given chemotherapy, randomized controlled trials of the causal effects of chemotherapy on neurocognitive function cannot be ethically conducted.21
Data extraction
Data extraction was performed using standardized forms by the first author and reviewed by the other authors. Data extracted included study design, the number of patients and controls, patient and control group characteristics (age at diagnosis and evaluation, time since diagnosis, length of follow-up), and the treatment protocol or regimen, when available. Data on 11 prespecified outcomes of interest were extracted when available: Full Scale intelligence quotient(FSIQ), Verbal IQ (VIQ), Performance IQ (PIQ), working memory, attention/concentration, information processing speed, verbal memory, visual memory, fine motor functioning, visuoconstruction, and executive function. It is important to note that these domains are not completely independent from each other as working memory and processing speed measurements are largely derived from the Wechsler IQ scales. Thus, scores and results of these outcomes are not truly independent of the IQ results. Intelligence is considered an integration of different neurocognitive functions.23 When studies reported results for multiple time points, the latest time point was analyzed. In instances when data were not available, multiple attempts were made to contact the study authors to retrieve this additional information.
Statistical analysis
All data were analyzed using Review Manager 5 (The Cochrane Collaboration, Copenhagen, Denmark). All extracted data for outcomes of interest were continuous and included mean score and standard deviation (SD). Weighted mean differences were calculated when the same instrument was used by all studies for a neurocognitive outcome, and standardized mean differences (SMDs) were calculated when studies used different measures. A fixed-effect inverse variance model was used; within-study variance was used to calculate the weight of each study.24 Heterogeneity was assessed using an I2 test, with an I2 test result greater than 50% indicating moderate to high heterogeneity.24,25 I2 measures the degree of variability in an analysis that is due to heterogeneity rather than chance.25 Quality of study and risk of bias were evaluated for all studies based on both Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines20 and Cochrane Collaboration Grades of Recommendation, Assessment, Development and Evaluation criteria.25 Sensitivity analyses were applied for all outcomes in all studies, particularly for those studies perceived to have a higher risk of bias because of methodological deficiencies and for results with a high degree of heterogeneity (I2 ≥ 75%).25 All measures were reported with 95% confidence intervals (CIs). Negative values indicate neurocognitive deficiencies in the ALL survivor group compared with the healthy control group. All statistical tests were 2-sided, and statistical significance was defined as P < .05.
Results
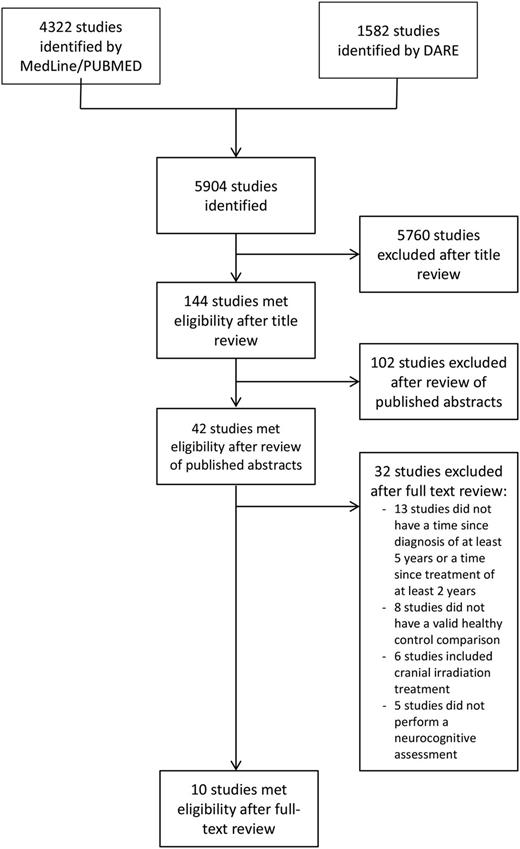
In total, 5904 titles and abstracts were identified in the initial broad search. After eliminating duplicate titles and studies that did not pertain to ALL survivors and neurocognitive outcomes, 144 articles remained. A review of abstracts was performed to screen whether they met our inclusion criteria, leaving 42 articles for full text review. Of the 42 studies, 13 were excluded because patients were less than 5 years since diagnosis or 2 years since the end of treatment, 8 because they lacked a healthy control group, 6 because some/all patients received cranial radiation, and 5 because they did not directly assess neurocognitive outcomes of interest (summarized in Figure 1). Ten studies11,19,26-34 remained that met all the predefined inclusion criteria (Table 1).
Study flow diagram. DARE, Database of Abstracts of Reviews of Effects.
Characteristics of included studies
| Study . | Country . | No. control subjects . | No. ALL survivors . | Mean age at diagnosis, y* . | Mean time since diagnosis, y* . | Mean age at final evaluation, y* . | ALL treatment protocol . |
|---|---|---|---|---|---|---|---|
| 19† | UK | 132 | 289 | 5.5 | 5.6 | 10.0 | MRC-UKALL XI |
| 27 | Finland | 45 | 20 | 6 | 17 | 23 | NOPHO ALL 1992 |
| 28 | US | 10 | 10 | 3.3 | 5.3 | 10.3 | Modified ALinC #12 (SWOG) |
| 29†,‡ | Netherlands | 28 | 40 | 6.4 | NR | 11.2 | DCOG ALL-9 |
| 30†,‡ | Netherlands | 225 | 17 | 3.5 | NR | 9.3 | DCLSG ALL VI |
| 11‡ | Netherlands | 225 | 20 | 3.4 | 7.2 | 10.3 | DCLSG ALL VII |
| 31 | Norway | 35 | 35 | 3.8 | 7.7 | 11.5 | NOPHO ALL 1992 |
| 32‡ | New Zealand | 21 | 21 | 4.1 | NR | 9.4 | ANZCCSG V and VI |
| 33 | Norway | 42 | 40 | 4.0 | 7.9 | 11.8 | NOPHO ALL 1992 |
| 34 | UK | 17 | 17 | 4.0 | 5.0 | 9.7 | MRC-UKALL XI |
| Study . | Country . | No. control subjects . | No. ALL survivors . | Mean age at diagnosis, y* . | Mean time since diagnosis, y* . | Mean age at final evaluation, y* . | ALL treatment protocol . |
|---|---|---|---|---|---|---|---|
| 19† | UK | 132 | 289 | 5.5 | 5.6 | 10.0 | MRC-UKALL XI |
| 27 | Finland | 45 | 20 | 6 | 17 | 23 | NOPHO ALL 1992 |
| 28 | US | 10 | 10 | 3.3 | 5.3 | 10.3 | Modified ALinC #12 (SWOG) |
| 29†,‡ | Netherlands | 28 | 40 | 6.4 | NR | 11.2 | DCOG ALL-9 |
| 30†,‡ | Netherlands | 225 | 17 | 3.5 | NR | 9.3 | DCLSG ALL VI |
| 11‡ | Netherlands | 225 | 20 | 3.4 | 7.2 | 10.3 | DCLSG ALL VII |
| 31 | Norway | 35 | 35 | 3.8 | 7.7 | 11.5 | NOPHO ALL 1992 |
| 32‡ | New Zealand | 21 | 21 | 4.1 | NR | 9.4 | ANZCCSG V and VI |
| 33 | Norway | 42 | 40 | 4.0 | 7.9 | 11.8 | NOPHO ALL 1992 |
| 34 | UK | 17 | 17 | 4.0 | 5.0 | 9.7 | MRC-UKALL XI |
ANZCCSG, Australian and New Zealand Children’s Cancer Study Group; DCLSG, Dutch Childhood Leukemia Study Group; DCOG, Dutch Childhood Oncology Group; MRC-UKALL, Medical Research Council Acute Lymphoblastic Leukemia Trial; NOPHO, Nordic Society of Pediatric Hematology and Oncology; NR, not reported; SWOG, Southwestern Oncology Group; UK, United Kingdom; US, United States.
A median value is provided if the mean was not available.
Longitudinal study in which data from the last time point was used.
Not all age data were reported.
A total of 509 survivors and 555 controls were included in these studies. Six of the 10 studies used a cross-sectional design, identified participants at some point after remission was established, and conducted at least 1 neurocognitive assessment after completion of treatment.27,28,31-34 The other 4 studies used a longitudinal design, identified patients at diagnosis, and completed periodic neurocognitive assessments during treatment and off therapy.11,19,29,30 Collectively, the study population included in the analyses had a mean age at diagnosis of 4.4 years (median, 4.0), a mean age at last evaluation of 11.7 years (median, 10.3), and a mean time since diagnosis of 8.0 years (median, 8.2). Four studies had a mean time since diagnosis of less than 6 years.29,30,32,34 Most studies excluded ALL survivors with documented premorbid learning disabilities or other developmental conditions, such as Down syndrome.11,27-31,33
In most studies, healthy controls were selected from the surrounding community. Two studies used siblings within the same age group as their healthy controls,29,34 whereas 4 other studies11,28,30,31 used controls matched on age, gender, socioeconomic status, and handedness (Kingma et al11 only). No study found significant differences between the ALL group and the control group in age and gender, although there was some variation in socioeconomic status.
In the 10 studies, ALL survivors had received therapy with chemotherapy only. Treatment protocols varied by study, but always included systemic corticosteroids as well as intrathecal and systemic methotrexate. Six of the studies conclusively showed that ALL survivors experienced deficits in neurocognitive functioning when compared with age-matched healthy controls (Table 1), which was attributed to their chemotherapy-only treatment.19,28,31-33 The other 4 studies found either very small differences or differences that were not statistically significant when compared with the healthy control group.
Intelligence (FIQ, VIQ, PIQ)
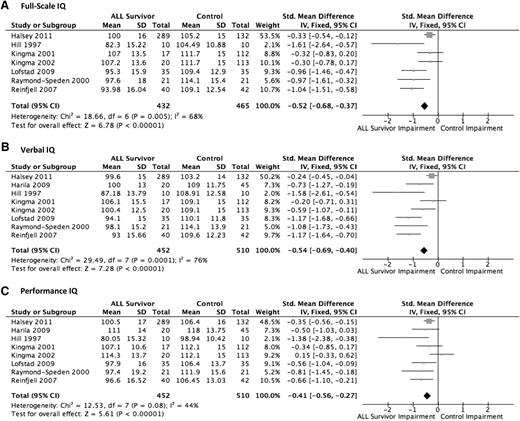
Intelligence or overall cognitive ability was measured by 8 studies.11,19,28,30-33 IQ was measured and assessed across 3 dimensions of the Wechsler Intelligence Scales (version dependent on age of patient): FSIQ, VIQ, and PIQ. For FSIQ, 432 ALL survivors met eligibility to be included in the treatment group and were compared with 465 healthy control subjects. For VIQ and PIQ, 452 pediatric ALL survivors were compared with 510 healthy control subjects.
For FSIQ (Figure 2A), the pooled estimate of the 7 relevant trials showed that the ALL survivor group scored significantly lower than the control group (P < .001). In every study, the ALL group scored lower than the control group at varying levels. Aggregately, using SMDs, the treatment group scored −0.52 SD (95% CI, −0.68 to −0.37) lower than the healthy control group, equivalent to 7.8 points lower.
For VIQ (Figure 2B), the meta-analysis of the 8 relevant trials showed that the ALL survivor group performed significantly worse than the healthy control group (P < .001) overall and for each of the studies. When these data were pooled using SMDs, on average the treatment group scored −0.54 SD (95% CI, −0.69 to −0.40) worse on the VIQ test, equivalent to 8.1 IQ points lower. High heterogeneity was noted (I2 = 76%).
The results for PIQ (Figure 2C) were less pronounced, but still statistically significant. Seven of the 8 studies showed lower PIQ in the ALL survivor group compared with the healthy control group. One study found the treatment group performed slightly better, although the difference was small.11 Overall, the pooled estimate, using SMDs, showed a significantly lower score for the ALL survivors (P < .001) of −0.41 SD (95% CI, −0.56 to −0.27) compared with the control group, equivalent to 6.15 IQ points lower.
Across all measures of IQ, the ALL survivor group performed worse than the healthy control group. Sensitivity analyses for each of the 3 outcomes yielded nearly identical results when individual studies were excluded, with no significant changes in overall effect size or statistical significance.
Working memory
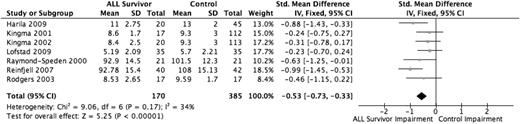
Working memory is the temporary storage and manipulation of information. Seven studies evaluated working memory using the Wechsler Digit Span test,11,27,30,34 Wechsler Digit Span Backward test,31 and Wechsler Freedom from Distractibility Index32,33 (version dependent on age of patient), for a total sample size of 555 individuals (170 ALL survivors, 385 healthy controls). All 7 studies found worse working memory functioning among the ALL survivors compared with the healthy control group. The pooled estimate (Figure 3), using SMDs, demonstrated that the ALL survivors performed −0.53 SD (95% CI, −0.73 to −0.33) lower than the healthy control group (P < .001). Sensitivity analyses did not show significant differences in the results; all modified models showed a similar deficiency among ALL survivors and maintained statistical significance.
Attention/concentration
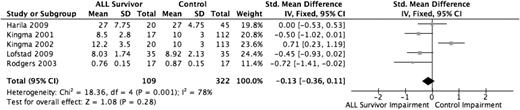
Five studies evaluated the domain of attention/concentration using the Bourdon-Wiersma Attention Test - Accuracy,11,30 the Trail Making Test Part A,27 the vigilance (VIGIL) Hit Rate score,34 and the Wechsler Digit Span Forward Test.32 The analysis included 109 ALL survivors and 322 healthy controls. Three of the 5 included studies found decreased attention and concentration functioning among the ALL survivor group,30,31,34 whereas 1 study found the ALL survivor group scored higher than the healthy control group.11 The overall pooled effect estimate (Figure 4) was not found to be statistically significant (P = .28).
Information processing speed
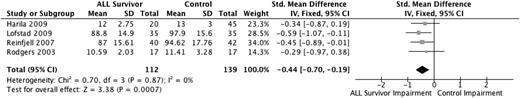
Information processing speed is the speed of mental processing. Four studies evaluated information processing speed using the Wechsler Processing Speed Index27,31,33,34 (version dependent on age of patient), with a total sample size of 251 individuals (112 ALL survivors, 139 healthy controls). All 4 studies found diminished functioning among ALL survivors, with the pooled estimate (Figure 5), using SMDs, revealing that the ALL survivors performed −0.44 SD (95% CI, −0.70 to −0.19) lower than the healthy control group (P < .001). Sensitivity analyses, excluding the study perceived to have some methodological weaknesses,34 increased the deficiency in ALL survivors to −0.47 SD (95% CI, −0.74 to −0.19) (P < .001).
Verbal and visual memory
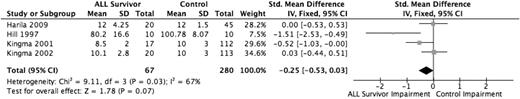
Four studies evaluated verbal memory, the ability to encode and retrieve verbally presented information,11,27,28,30 in a total sample of 67 ALL survivors and 280 healthy controls. Verbal memory was evaluated using the Rey Auditory Verbal Learning Test–Delayed Recall,11,30 the Wide Range Assessment of Memory and Learning Verbal Memory Index,28 and the Wechsler Memory Scale–Logical Memory–Delayed.27 Only 2 of the studies found diminished verbal memory functioning in the ALL survivor group compared with the healthy control group.28.30 When the 4 studies were pooled (Figure 6) using SMDs, the overall effect estimate approached significance (P = .07), with the ALL group scoring −0.25 SD (95% CI, −0.53 to 0.03) lower than the control group. Sensitivity analysis removing the study with increased risk of bias (Harila et al27 ) decreased the scoring gap of the ALL group, −0.15 SD (−0.44 to 0.14) and was not statistically significant (P = .30).
Only 1 study28 measured visual memory, the ability to encode and retrieve nonverbal information. ALL survivors scored significantly lower on the Wide Range Assessment of Memory and Learning Visual Memory Index (P < .05), but the study had a small sample size (n = 20).
Fine motor functioning
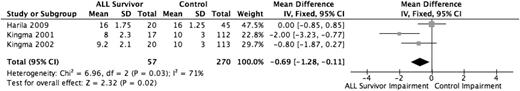
Three studies evaluated fine motor functioning,11,27,30 all with the same version of the Purdue Pegboard test, with a total sample of 57 ALL survivors and 270 healthy controls. Using weighted mean differences, the pooled effect estimate (Figure 7) showed that the ALL group performed worse (SD, −0.69; 95% CI, −1.28 to −0.11) than the healthy control group (P = .02). The low number of studies precluded us from conducting a meaningful sensitivity analysis.
Visuoconstruction
Insufficient data precluded calculating a pooled estimate of visuoconstruction, a skill that combines visual perceptual activity with a motor response. Three studies included in the analysis evaluated this domain11,29,30 with the Beery Visual Motor Integration Test. Only 1 study found a statistically significant impairment in visuoconstruction functioning among the ALL survivor group when compared with the healthy control group.29 The other 2 studies found slightly worse functioning in the ALL survivor group30 and slightly better functioning among the ALL survivor group,11 with neither result being statistically significant.
Executive function
Four studies11,27,29,34 evaluated executive function, the ability to plan, organize, sequence, and manage multiple tasks simultaneously.35 Insufficient study data precluded estimating a pooled effect. This domain was evaluated with the Trail Making Test Part B11,27 and the Wisconsin Card Sorting Test.29,34 Only 1 study found a statistically significant decrease in executive functioning among the ALL survivors,11 whereas the other 3 found nonsignificant decreases among the ALL group.
Discussion
This systematic review and meta-analysis demonstrated moderate long-term neurocognitive impairment occurring after ALL treatment without cranial radiation. Intelligence was most affected, with a reduction of 6 to 8 IQ points as compared with healthy controls. Working memory and information processing speed were also moderately affected with impairments of about half of an SD. Impairment in fine motor functioning was also quite pronounced, showing a statistically significant decrement of about three-fourths of an SD among ALL survivors. This consistent impairment among ALL survivors across multiple domains indicates that children treated with chemotherapy-only regimens experience clinically significant neurocognitive impairment.15-17
Contrary to previous systematic reviews, we found a substantial decrease in IQ as well as moderate impairment in other domains. Although one prior review found impairment in intelligence immediately following treatment,17 our review is the first to evaluate long-term impairment and to report the decreases in IQ and other scores. Although the majority of existing literature has focused on the overall cognitive functioning, as measured by the Wechsler IQ scale, and academic achievement assessed closer to the time of diagnosis,13,17 newer studies have started to examine more specific neurocognitive domains. The results of these studies have been mixed, although many have shown decreased functioning in cognitive domains such as working memory and attention.15,36,37 These findings, paired with our results, indicate that intelligence, as a measure of overall cognitive function, and several individual neurocognitive domains may be affected.
We did not find a significant decrement in attention/concentration, which has been described in other studies of pediatric leukemia treated with chemotherapy only.16,18,38 It is possible that our null results were due to the challenges of measuring a complex construct such as attention, which was variably defined and assessed in the included studies. Consistent with our study, however, other researchers have also found subtle39 or no deficitis40,41 in studies that were not included in our meta-analysis.
The mechanism of neurocognitive impairments from chemotherapy-only treatment is likely related to the specific chemotherapy agents included in treatment protocols that are associated with neurotoxicity.42 Methotrexate is associated with white matter changes in the brain,43 acute seizures and paralysis,44,45 and neurocognitive changes.46 Corticosteroids can result in behavior changes47 and decreased hippocampal functioning.48 Vincristine can cause peripheral neuropathy.49 Although these drugs are usually given orally and intravenously, standard state-of-the-art therapy for ALL also includes periodic intrathecal injections of methotrexate and corticosteroids directly into the CNS, which may further exacerbate toxicity.50
We acknowledge potential limitations of this meta-analysis. For some neurocognitive domains, the sample size of the pooled estimates was small. However, it represents the entirety of the published literature in this area. The relative lack of studies overall, as well as in specific neurocognitive domains, indicates the need for further research. For instance, though we included achievement-related terms in our search criteria, no studies met our inclusion criteria; therefore, we were unable to analyze this important outcome. Thus, aggregation of the data offers advantages over any single article in improving statistical power. However, the variability in measurements used to evaluate certain neurocognitive domains, such as attention/concentration and verbal memory, may be an alternative explanation for why some areas of functioning were not significantly impaired. Similarly, although we found a moderate decline in working memory over time, this analysis was potentially confounded by our inability to parse out tests that are arguably not the strongest assessment of that function. Thus, these results should be interpreted cautiously. Additionally, it is important to acknowledge that the construct of intelligence is broad and variably defined in different instruments. Numerous lower and higher order cognitive functions contribute to performance on the Wechsler IQ test. Though this analysis indicates a decrement in intelligence over time, beyond the areas analyzed, it is unclear what specific neurocognitive areas may be contributing to this decline in performance. This is particularly important because the optimal targets for potential intervention are similarly unclear.
Because of the limited size and number of studies meeting eligibility criteria, we were unable to examine some important potential risk factors such as age at diagnosis, gender, and chemotherapy exposures, which could moderate the effects on outcomes. These critical demographic and medical factors have been correlated with ALL outcomes in the past29,51 and may be correlated with neurocognitive function.29 Furthermore, our study did not have the available data to address whether there was improvement in neurocognitive functioning with time. To limit the measurement of acute effects of chemotherapy, we only included studies of patients who were at least 2 years off therapy or 5 years after diagnosis. Previous studies suggest that neurocognitive deficits get worse over the first several years posttherapy and do not improve with time.6,27
Because of the small number of studies, we could not evaluate publication bias via visual inspection using funnel plots to produce a meaningful interpretation.52 Therefore, publication bias is certainly a limitation of our meta-analysis, particularly because we were unable to evaluate unpublished data related to our outcomes. However, this type of publication bias is a limitation of all research and is not unique to our study.53-57 One potential source of bias in our included studies is volunteer or survivor bias because most studies had at least some ALL survivors who chose not to participate. Most studies provided evidence for why volunteer bias likely did not affect their results, but this was not the case in 4 studies.28,31,32,34 Because the results of these 4 studies were similar to the other studies, any bias likely did not meaningfully influence the effect estimates. Similarly, bias from follow-up attrition seemed to be minimized because nearly all participants received assessments at all time points. When the participation rate in 1 study seemed low for the final time point19 included in our analysis, it did not differ significantly from earlier time point participation rates. Furthermore, the sensitivity analyses did not yield substantially different results when these studies with potential bias issues were excluded. Generally, by selecting only studies that included a control group, some degree of selection bias is introduced. These studies tend to be better funded and more complex. However, to address the aims of our meta-analysis, studies with control groups were required to make a more accurate assessment of neurocognitive deficits. Pediatric ALL incidence and survival vary greatly by race and socioeconomic status.1,56,57 Therefore, control group results would be more valid than standardized norms to assess neurocognitive impairment in this population.
Despite these limitations, our results support surveillance of neurocognitive effects in ALL survivors treated without radiation because they appear to be at significantly greater risk for cognitive deficits. Special considerations should be given to providing appropriate educational planning, including putting supportive interventions in place as early as possible to avoid risk of long-term educational failures. One previous study found that although childhood leukemia survivors were less likely to graduate from high school compared with nonaffected sibling controls, when these survivors received special education services, differences in graduation rate were attenuated.58 Thus appropriate monitoring of neurocognitive function and intervening when impairments are detected are key to mitigating the long-term effect of cancer therapy in this patient population. Pharmacological and nonpharmacological interventions for remediating problems in working memory,49 attention,59,60 and executive function61,62 continue to be investigated and show promise for the future.
Although our results provide important answers to the issue of long-term neurocognitive effects of chemotherapy-only treatments in pediatric ALL survivors, and build on previous literature, they also highlight key gaps in research. Studies are needed to address the long-term effects on individual domains of neurocognitive function, outside of intelligence, to provide a clearer picture of the totality of the dysfunction that can result from chemotherapy-only treatment in this population. Additionally, given progress already made in quantifying these long-term deficiencies, further research is needed to evaluate the implications of these moderate impairments on life achievements, including level of educational attainment, employment, and independence of living. Preventive strategies should be researched, including identifying inherited genetic variants that may predict vulnerability to neurocognitive therapy63 and studying ways to minimize therapy without compromising remission and cure, especially for lower risk ALL subtypes.
In summary, the results of this meta-analysis strongly support an association between chemotherapy-only treatment of pediatric ALL and long-term neurocognitive deficiencies, particularly in intelligence, working memory, and information processing speed. Although current treatment regimens are associated with high cure rates, our results emphasize the need for future research and guidance for clinicians to improve long-term outcomes for these patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This study was supported in part by research funding from the St. Baldrick’s Foundation (to N.S.K.-L.).
Authorship
Contribution: N.S.I., L.M.B., M.B.B., and N.S.K.-L. participated in the design of the study; N.S.I. conducted the literature search, which was verified and confirmed by L.M.B. and N.S.K.-L.; N.S.I. and M.B.B. performed all statistical analyses; and N.S.I. wrote the paper, which was reviewed and edited by L.M.B., M.B.B., and N.S.K.-L.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nina S. Kadan-Lottick, Yale University School of Medicine, Section of Pediatric Hematology/Oncology, 333 Cedar St, LMP 2073, New Haven, CT 06520; e-mail: nina.kadan-lottick@yale.edu.