Abstract
Humans are now understood to be in complex symbiosis with a diverse ecosystem of microbial organisms, including bacteria, viruses, and fungi. Efforts to characterize the role of these microorganisms, commonly referred as the microbiota, in human health have sought to answer the fundamental questions of what organisms are present, how are they functioning to interact with human cells, and by what mechanism are these interactions occurring. In this review, we describe recent efforts to describe the microbiota in healthy and diseased individuals, summarize the role of various molecular technologies (ranging from 16S ribosomal RNA to shotgun metagenomic sequencing) in enumerating the community structure of the microbiota, and explore known interactions between the microbiota and humans, with a focus on the microbiota’s role in hematopoiesis and hematologic diseases.
Introduction
The microorganisms living in association with the human body are collectively called the microbiota, with most microbe-host interactions being symbiotic as opposed to pathogenic.1,2 There are ∼10 to 100 trillion microbes in a human; microbial cells and particles thus outnumber human cells at a ratio of 10:1.3 Microbes reside in and on many of the body’s surfaces, including the skin, nares, oropharynx, vagina, and intestine. The majority of human-associated microbes reside within the colon, and this microbial consortium is established within the first 3 years of life.4-7 Of these lower intestinal microbes, the majority of the bacteria are in 2 of the 70 phyla: Bacteroidetes and Firmicutes (Table 1).8
Major bacterial phyla represented in the human intestine
| Phyla . | Description . |
|---|---|
| Bacteroidetes | This phylum comprises aerobic and anaerobic Gram-negative bacteria, which commonly function to help break down carbohydrates. The breakdown products of carbohydrate metabolism have been shown to modify host inflammation. An example of an organism from this phylum is Bacteriodes fragilis. |
| Firmicutes | This phylum comprises Gram-positive bacteria with low levels of guanine and cytosine nucleotides. It contains bacteria that are phenotypically diverse and are able to form endospores. An example of an organism from this phylum is Roseburia hominis. |
| Actinobacteria | This phylum is primarily composed of Gram-positive bacteria with high guanine and cytosine nucleotide levels. These organisms are commonly associated with the production of secondary metabolites. An example of an organism from this phylum is Bifidobacterium bifidum. |
| Proteobacteria | This phylum is composed of Gram-negative bacteria, many of which are pathogenic to humans. In the gut microbiota of healthy individuals, Proteobacteria are classically believed to be less abundant than Bacteroidetes, Firmicutes, and Actinobacteria. However, bacteria in this phylum frequently expand in dysbiosis or other disease states. An example of an organism from this phylum is Escherichia coli. |
| Phyla . | Description . |
|---|---|
| Bacteroidetes | This phylum comprises aerobic and anaerobic Gram-negative bacteria, which commonly function to help break down carbohydrates. The breakdown products of carbohydrate metabolism have been shown to modify host inflammation. An example of an organism from this phylum is Bacteriodes fragilis. |
| Firmicutes | This phylum comprises Gram-positive bacteria with low levels of guanine and cytosine nucleotides. It contains bacteria that are phenotypically diverse and are able to form endospores. An example of an organism from this phylum is Roseburia hominis. |
| Actinobacteria | This phylum is primarily composed of Gram-positive bacteria with high guanine and cytosine nucleotide levels. These organisms are commonly associated with the production of secondary metabolites. An example of an organism from this phylum is Bifidobacterium bifidum. |
| Proteobacteria | This phylum is composed of Gram-negative bacteria, many of which are pathogenic to humans. In the gut microbiota of healthy individuals, Proteobacteria are classically believed to be less abundant than Bacteroidetes, Firmicutes, and Actinobacteria. However, bacteria in this phylum frequently expand in dysbiosis or other disease states. An example of an organism from this phylum is Escherichia coli. |
The most comprehensive efforts to catalog the composition and potential functions of various body sites’ microbiota have been conducted by the Human Microbiome Project (HMP), a consortium-based endeavor supported by the National Institutes of Health Common Fund, and the Metagenomics of the Human Intestinal Tract project supported by the European commission (definitions of common terms used in microbiota-related studies are provided in Table 24,9-15 ). High-throughput sequencing–based studies have been conducted to characterize human and microbial genes and genomes, with this collective combination of genes referred to as the metagenome.16 This information can then be used to help understand the microbial parts of our genetic and metabolic landscape.4
Glossary of terms related to microbiota studies
| Term . | Definition . | Reference . |
|---|---|---|
| Gnotobiology | The scientific investigation of organisms that are either free of microorganisms or are associated only with specified microorganisms. | 9 |
| Germ-free (axenic) mouse models | Mice free of all microorganisms, including those that are typically found in the gut. | 10 |
| Microbiota | The collection of all the microorganisms living in association with the human body. These communities consist of a variety of microorganisms including eukaryotes, archaea, bacteria and viruses. | 4 |
| Microbiome | Generally accepted to refer to the genomes (genetic material [DNA or RNA]) of the microbiota. | |
| Dysbiosis | A breakdown in the balance between putative species of “protective” vs “harmful” intestinal microorganisms. | 11 |
| Metagenomics | The study of a collection of genetic material (genomes) from a mixed community of organisms (ie, microbial communities). | |
| Monocolonization | Introduction of 1 bacterial species in axenic mice; alternatively, a clinical situation in which a single organism dominates the microbiota as a result of disease or treatment characteristics. | |
| Oligocolonization | Introduction of multiple bacterial species in axenic mice; alternatively, a clinical situation in which a small number (typically ∼2-5) of organisms dominate the microbiota as a result of disease or treatment characteristics. | |
| Fecal microbiota transfer | Introduction of a fecal suspension derived from a healthy donor into the gastrointestinal tract of a diseased individual. Fecal microbiota transfer can be performed via the introduction of an auto (self) or allo (other) source of stool or purified bacterial stool product via a multitude of methods, including administration by nasogastric tube, colonoscopy, or capsulized therapy. | 12 |
| Next-generation whole-genome sequencing | DNA is fragmented, amplified, and then sequenced in a massively parallel fashion. This sequence data can be compared to reference databases to infer the taxonomic community structure of a population of organisms; alternatively, novel organisms/strains can be discovered by de novo sequence assembly methods. | 13 |
| 16S ribosomal RNA/DNA sequencing | 16S ribosomal RNA/DNA of prokaryotes is amplified via polymerase chain reaction and sequenced to identify the genus or species of individual organisms. | 14 |
| Viral particle isolation and sequencing | Viral particles are separated from host contaminants using methods such as centrifugation and filtration. Viral particles can then be treated with DNase I to remove contaminating nucleic acids on the surface of these particles. A variety of molecular methods can be used to characterize the genomic sequence of the virions. | 15 |
| Term . | Definition . | Reference . |
|---|---|---|
| Gnotobiology | The scientific investigation of organisms that are either free of microorganisms or are associated only with specified microorganisms. | 9 |
| Germ-free (axenic) mouse models | Mice free of all microorganisms, including those that are typically found in the gut. | 10 |
| Microbiota | The collection of all the microorganisms living in association with the human body. These communities consist of a variety of microorganisms including eukaryotes, archaea, bacteria and viruses. | 4 |
| Microbiome | Generally accepted to refer to the genomes (genetic material [DNA or RNA]) of the microbiota. | |
| Dysbiosis | A breakdown in the balance between putative species of “protective” vs “harmful” intestinal microorganisms. | 11 |
| Metagenomics | The study of a collection of genetic material (genomes) from a mixed community of organisms (ie, microbial communities). | |
| Monocolonization | Introduction of 1 bacterial species in axenic mice; alternatively, a clinical situation in which a single organism dominates the microbiota as a result of disease or treatment characteristics. | |
| Oligocolonization | Introduction of multiple bacterial species in axenic mice; alternatively, a clinical situation in which a small number (typically ∼2-5) of organisms dominate the microbiota as a result of disease or treatment characteristics. | |
| Fecal microbiota transfer | Introduction of a fecal suspension derived from a healthy donor into the gastrointestinal tract of a diseased individual. Fecal microbiota transfer can be performed via the introduction of an auto (self) or allo (other) source of stool or purified bacterial stool product via a multitude of methods, including administration by nasogastric tube, colonoscopy, or capsulized therapy. | 12 |
| Next-generation whole-genome sequencing | DNA is fragmented, amplified, and then sequenced in a massively parallel fashion. This sequence data can be compared to reference databases to infer the taxonomic community structure of a population of organisms; alternatively, novel organisms/strains can be discovered by de novo sequence assembly methods. | 13 |
| 16S ribosomal RNA/DNA sequencing | 16S ribosomal RNA/DNA of prokaryotes is amplified via polymerase chain reaction and sequenced to identify the genus or species of individual organisms. | 14 |
| Viral particle isolation and sequencing | Viral particles are separated from host contaminants using methods such as centrifugation and filtration. Viral particles can then be treated with DNase I to remove contaminating nucleic acids on the surface of these particles. A variety of molecular methods can be used to characterize the genomic sequence of the virions. | 15 |
Host genetics play an important role in establishing and shaping the microbiota. Single-nucleotide polymorphisms in specific host genomic loci are believed to impact microbiota composition.17 In contrast, monozygotic-twin metagenomic studies have revealed notable variability in the composition of microbial communities in healthy individuals, with twins sharing <50% of their species-level bacterial taxa.18 This finding suggests a significant role for environmental and other factors in shaping microbiota community structures. Viral communities, for example, vary widely among monozygotic twins.19 Complex interplay between host and environmental factors is critical in shaping the microbiota, and our understanding of these relationships is evolving rapidly. Many of the mechanisms that define the interface between microbes and humans have been defined in the context of the gut microbiota–host relationship (Figure 1); however, our understanding of the full complexity of host-microbe interactions, even in this well-studied context, is incomplete. Further research will likely clarify the mechanistic relationship between the human host, microbial genes, and the microbiota.
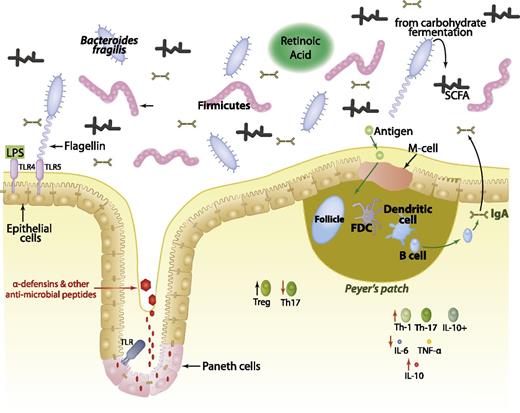
The role of the gut microbiota and associated products in shaping the intestinal immune system. Commensal organisms, such as bacteria in the phyla Bacteriodes and Firmicutes, colonize the gastrointestinal tract. SCFAs are produced as a result of carbohydrate fermentation, which increases the production of IL-10 and decreases the production of IL-6 and tumor necrosis factor (TNF)-α. Retinoic acid, which is produced by intestinal dendritic cells, leads to an increased differentiation of Tregs and decreased differentiation of inflammatory Th17 cells. The microfold (M)-cells of Peyer patches uptake antigen from the lumen and deliver it to the dendritic cells and other antigen-presenting cells located in the lamina propria. These cells become activated, and B cells secrete immunoglobulin (Ig)A into the lumen. These bacteria-specific IgA molecules thus serve to modulate the luminal microbiota composition. Paneth cells, an intestinal epithelial cell subtype that is prevalent in the small intestine and ascending colon, also shape the microbial composition by secreting α-defensins and other antimicrobial proteins in response to bacterial antigens binding to toll-like receptors (TLRs). TLRs line the gastrointestinal tract, and microbial products such as lipopolysaccharide (LPS) and flagellin bind to TLR4 and TLR5, respectively, upregulating the expression of RegIIIγ, a secreted antibacterial lectin that limits infection from Gram-positive bacteria. FDC, follicular dendritic cell; IL, interleukin; SCFA, short chain fatty acid; Treg, regulatory T cell; Th, helper T.
The role of the gut microbiota and associated products in shaping the intestinal immune system. Commensal organisms, such as bacteria in the phyla Bacteriodes and Firmicutes, colonize the gastrointestinal tract. SCFAs are produced as a result of carbohydrate fermentation, which increases the production of IL-10 and decreases the production of IL-6 and tumor necrosis factor (TNF)-α. Retinoic acid, which is produced by intestinal dendritic cells, leads to an increased differentiation of Tregs and decreased differentiation of inflammatory Th17 cells. The microfold (M)-cells of Peyer patches uptake antigen from the lumen and deliver it to the dendritic cells and other antigen-presenting cells located in the lamina propria. These cells become activated, and B cells secrete immunoglobulin (Ig)A into the lumen. These bacteria-specific IgA molecules thus serve to modulate the luminal microbiota composition. Paneth cells, an intestinal epithelial cell subtype that is prevalent in the small intestine and ascending colon, also shape the microbial composition by secreting α-defensins and other antimicrobial proteins in response to bacterial antigens binding to toll-like receptors (TLRs). TLRs line the gastrointestinal tract, and microbial products such as lipopolysaccharide (LPS) and flagellin bind to TLR4 and TLR5, respectively, upregulating the expression of RegIIIγ, a secreted antibacterial lectin that limits infection from Gram-positive bacteria. FDC, follicular dendritic cell; IL, interleukin; SCFA, short chain fatty acid; Treg, regulatory T cell; Th, helper T.
Historically, the microbiota focus has been on bacteria; however, viruses, bacteriophages, and fungi also contribute to the ecological diversity in several anatomical niches in humans. The best studied of these 3 additional components of the microbiota are eukaryotic viruses (and to a lesser extent, prokaryotic viruses, which are known as bacteriophages). Eukaryotic viruses are posited to contain a staggering amount of nucleic acid and amino acid sequence diversity.20 The identification of known and novel viruses has been potentiated by the development and use of sequencing-based techniques. Sequencing is typically followed by computational analysis using programs designed to identify and phylogenetically characterize viruses.21 Interestingly, interplay between the bacterial and viral components of the microbiota has been reported; in a study of the infectivity of poliovirus in a mouse model, treatment of the mice with antibiotics prior to poliovirus exposure decreased host susceptibility to disease.22 Abnormalities in the enteric virome are associated with inflammatory bowel disease, with an increase in enteric virome richness in Crohn disease and ulcerative colitis.20 This association suggests a potential relationship between viruses of the microbiota and host inflammation; the nature of the mechanistic interactions between the virome and the immune system need to be further elucidated. Although prokaryotic viruses have been genomically enumerated in the human microbiota, the understanding of bacteriophage regulation is in its relative infancy.
Advances in molecular technology
Advances in biological techniques have potentiated great advances in understanding the human microbiota. Studies of the microbiota began with microscopy and culturing of organisms. As polymerase chain reaction and Sanger sequencing became widely used, multilocus sequence typing (MLST) gained popularity. In this technique, ∼470-bp fragments from several housekeeping genes are amplified and sequenced to genotypically classify bacterial species.23 This method has been developed for a handful of pathogenic organisms and has been helpful in characterizing the relatedness of organisms that have been isolated and cultured from disease outbreaks. However, it is inherently limited by its inability to comprehensively identify organisms that lack defined sequences for MLST or are undiscovered. Approximately 60% to 70% of the bacteria in the human intestinal tract cannot be cultured with currently available methods.24 These so-called unculturable organisms can often be identified by 16S ribosomal (r) RNA sequence analysis.25 The phylogenetic analysis of bacterial 16S rRNA genes (ribosomal DNAs), amplified directly from complex communities, thus provides an efficient strategy for exploring the biodiversity of a particular biota.24 Recent data from an analysis of 16S rRNA sequencing revealed that 62% of the 395 bacterial phylotypes were novel and ∼80% of the RNA sequences were from previously uncultivated species.8 16S rRNA sequencing formed the core of the HMP microbiota characterization efforts, but it has limitations that include biases introduced by copy-number variations and challenges in delineation of closely related prokaryotic species.26 Several different bacterial species may share identical regions of the16S rRNA sequence, thus making definitive species-level taxonomic identification impossible. This limitation has recently been overcome by the application of high-throughput shotgun sequencing, which has helped to create reference genome databases for newly isolated and cultured organisms and has facilitated the discovery of near full genomes of novel, previously uncultured organisms in mixed samples.27,28 Shotgun sequencing (for whole genome sequencing of isolates and characterization of metagenomic samples) has become more accessible as the cost of sequencing has decreased, and was employed in a subset of samples characterized by the HMP; additionally, this method has been more broadly applied in more recent studies. Although this is a relatively accurate method for taxonomic identification of microbial components of a mixed population, it is computationally and time intensive (often requiring “supercomputing” infrastructure) and is often underpowered to reliably identify rare members of the microbial community. Excellent high-throughput, computationally streamlined methods exist to estimate the identity and relative abundance of microbial organisms within a metagenomic sample. For example, metagenomic analysis software programs such as MetaPhlAn expand on the concept of MLST by identifying hundreds of conserved or “marker” proteins so that taxonomic composition within a metagenomic population can be inferred.29 This and related technologies have been broadly applied in the research setting and are now expanding to the clinical setting where the ability to rapidly and accurately classify microbial organisms has demonstrated an impact on clinical outcomes.30
Microbiota in human health and disease
Our understanding of the role of the microbiota in human health and disease is built on epidemiological, observational, molecular, and animal-modeling experiments. The microbiota plays an important role in human health and disease. The microbiota is involved in energy harvest and storage, as well as in a variety of metabolic functions such as fermenting and absorbing undigested carbohydrates.31 In particular, the microbiota shapes global immune cell repertoires, thereby altering host susceptibility to inflammation and infection at sites of colonization (Figure 1).1,32 Most studies of the microbiota have been performed in axenic mice (see Table 2 for definition). Recent twin studies have demonstrated that the environment is a potent regulator of immune development, and this may include contributions from the microbiota.33 It is now well accepted that essential developmental functions of mammals, such as proper training and maturation of the immune system, require the presence of beneficial microorganisms, which are known as commensals.34
Dysbiosis, or an imbalance in microbiota composition, is postulated to be a major factor in a variety of human diseases, such as inflammatory bowel disease, graft-versus-host disease (GVHD), and acute HIV infection.35 In a now classic study, adult germ-free mice were colonized by the microbiota of obese mice or lean mice; the mice colonized with an obese microbiota had a significantly greater percentage increase in total body fat 2 weeks later.9 As has been clearly demonstrated, the microbiota is critical for the proper maturation of immune cells and tissues, and plays a role in protection against infectious pathogens. Furthermore, certain gut microbiota compositions are associated with disorders of autoimmunity, including inflammatory bowel diseases, diabetes, asthma, and allergy.34,36,37
Although associations between the microbiota and products of the hematopoietic system are well established, a mechanistic understanding of how the microbiota directly or indirectly impact hematopoiesis is limited. The size of the bone marrow myeloid cell pool has been shown to correlate strongly with the complexity of the intestinal microbiota.38 The immune system begins to develop in utero, but full maturation requires both genetic and environmental signals that further shape immunity after birth.39 Germ-free mice have an immature mucosal immune system, with reduced secondary lymphoid tissues, lower levels of secretory immunoglobulin A, and fewer intestinal plasma cells.2 In addition, germ-free mice have lower levels of serum antibodies and are more susceptible to intestinal and systemic bacterial infections.40 Certain key intestinal compartments, including lymph nodes, Peyer patches, and intestinal lymphoid follicles, only fully develop after birth when they undergo a programmed population change to cope with the microbiota and maintain homeostasis.41
Effects of microbial metabolites on hematopoiesis
Specific gut bacteria or bacterial products directly suppress intestinal inflammation (eg, colitis) in mice through a variety of immune mechanisms.42-44 Bacterial byproducts in the gut microbiome can also impact allergic airway disease by way of interaction of bacterial metabolites with cells of the bone marrow compartment.45
SCFAs serve many important roles, from regulating ion absorption and gut motility in the intestine, to modulating immune responses.46 SCFAs achieve these widespread functions by activating several G protein–coupled cell surface receptors, such as GPR43, which is expressed by granulocytes and some myeloid cells, and GPR109a, a receptor for niacin and C4 expressed by gut epithelial cells, adipocytes, macrophages, and dendritic cells. SCFAs suppress nuclear factor κB and the production of inflammatory cytokines such as IL-6 and tumor necrosis factor α, except for IL-10, which increases.47 These molecules increase generation of Th1, Th17, and IL-10+ cells but decrease the proliferation of T cells and B cells in the mouth.46,48 Butyrate, a type of SCFA, enhances T-cell apoptosis and decreases accumulation of T cells in inflamed colonic mucosa by upregulating Fas.49 Retinoic acid, a metabolite of dietary vitamin A, can promote induced Treg generation and inhibit Th17 and type 1 regulatory T–like cell responses.35 Polysaccharide A, a carbohydrate produced by the human commensal bacterium Bacteroides fragilis, is sufficient to ameliorate T cell–driven colitis in an IL-10–dependent manner.32
Given the evidence supporting a role for the microbiota in normal hematopoiesis, it is unsurprising that alterations in the microbiota, as well as in specific microbes, are associated with hematologic disorders. In some cases, pathogens are believed to trigger hematologic disorders; in others, disruption of homeostasis in the microbiota is associated with clinically significant outcomes in patients with hematologic disorders. Microbial organisms have been demonstrated, in some way, to impact every compartment of the hematopoietic system. Here, we outline major hematologic disease and describe associations with infections and/or alterations of the microbiota (Table 350-65 ).
Described associations between specific microorganisms, the microbiota, and hematologic disorders
| Disease . | Implicated microbiota . | Reference . |
|---|---|---|
| Aplastic anemia | Aplastic anemia has been reported after infections with human parvovirus B19; hepatitis A, B, C, E, and G; CMV; EBV; echovirus 3; GB virus C; transfusion-transmitted or Torque teno virus; SEN virus; and non–A-E hepatitis viruses. | 50-52 |
| Megaloblastic anemia | Although changes in the microbiota have not yet been directly associated with this disease, cyanocobalamin (vitamin B12) is synthesized by several genera of intestinal bacteria. | 53 |
| Anemia of chronic inflammation | Hepcidin is a gene that is induced by infection and inflammation. Hepcidin-dependent changes in iron flux can induce anemia in chronic inflammatory states. | 54 |
| Gastric MALT lymphoma | Linked to infection with Helicobacter pylori. | 55 |
| Marginal zone lymphomas | HCV might provide the initial antigenic stimulus for B-cell clonal expansion. | 56 |
| HTLV-associated acute T-cell leukemia | Etiologically linked to HTLV-1 and with a distinct geographical distribution. | 57 |
| Burkitt lymphoma | A subset of the cases of this aggressive lymphoma, especially endemic cases in sub-Saharan Africa, is associated with EBV infection. | 58 |
| PTLD | PTLD after solid organ transplant and HCT is associated with EBV, which leads to uncontrolled B-cell proliferation and tumor formation. | 59 |
| Castleman disease | Human herpesvirus 8 (Kaposi sarcoma–associated herpesvirus) sequences have been described in some cases of multicentric Castleman disease. | 60 |
| ITP | Often secondary to persistent, often inapparent infections (eg, H pylori, CMV, or HCV). | 61 |
| Reactive thrombocytosis | Cytokines, such as IL-6, IL-1, and tumor necrosis factor, have been shown to promote in vivo and in vitro megakaryocytopoiesis, or production of platelets. Any inflammatory process such as bacterial infection, neoplasia, sepsis, multiple trauma, burns, or pancreatitis that elevates serum interleukin levels (especially IL-6), may increase the circulating platelet count. | 62 |
| GVHD | Loss of Paneth cells from GVHD results in decreased production of α-defensins. α-Defensins selectively kill noncommensal bacteria while preserving commensal microbiota. A decrease in the expression of α-defensins may thus result in the loss of microbial diversity. | 63 |
| Allo-HCT | The gastrointestinal mucosa is damaged, and colonizing bacteria are impacted, leading to an impaired intestinal microbiota with reduced diversity. The diversity of the intestinal microbiota at engraftment is an independent predictor of mortality in allo-HCT recipients. Sequencing of free DNA in organ transplant recipients’ plasma revealed an expansion of Anelloviridae upon immunosuppression. There was a lower anellovirus burden in patients who suffer from graft rejection. A similar finding would be expected in the HCT population. | 64, 65 |
| Disease . | Implicated microbiota . | Reference . |
|---|---|---|
| Aplastic anemia | Aplastic anemia has been reported after infections with human parvovirus B19; hepatitis A, B, C, E, and G; CMV; EBV; echovirus 3; GB virus C; transfusion-transmitted or Torque teno virus; SEN virus; and non–A-E hepatitis viruses. | 50-52 |
| Megaloblastic anemia | Although changes in the microbiota have not yet been directly associated with this disease, cyanocobalamin (vitamin B12) is synthesized by several genera of intestinal bacteria. | 53 |
| Anemia of chronic inflammation | Hepcidin is a gene that is induced by infection and inflammation. Hepcidin-dependent changes in iron flux can induce anemia in chronic inflammatory states. | 54 |
| Gastric MALT lymphoma | Linked to infection with Helicobacter pylori. | 55 |
| Marginal zone lymphomas | HCV might provide the initial antigenic stimulus for B-cell clonal expansion. | 56 |
| HTLV-associated acute T-cell leukemia | Etiologically linked to HTLV-1 and with a distinct geographical distribution. | 57 |
| Burkitt lymphoma | A subset of the cases of this aggressive lymphoma, especially endemic cases in sub-Saharan Africa, is associated with EBV infection. | 58 |
| PTLD | PTLD after solid organ transplant and HCT is associated with EBV, which leads to uncontrolled B-cell proliferation and tumor formation. | 59 |
| Castleman disease | Human herpesvirus 8 (Kaposi sarcoma–associated herpesvirus) sequences have been described in some cases of multicentric Castleman disease. | 60 |
| ITP | Often secondary to persistent, often inapparent infections (eg, H pylori, CMV, or HCV). | 61 |
| Reactive thrombocytosis | Cytokines, such as IL-6, IL-1, and tumor necrosis factor, have been shown to promote in vivo and in vitro megakaryocytopoiesis, or production of platelets. Any inflammatory process such as bacterial infection, neoplasia, sepsis, multiple trauma, burns, or pancreatitis that elevates serum interleukin levels (especially IL-6), may increase the circulating platelet count. | 62 |
| GVHD | Loss of Paneth cells from GVHD results in decreased production of α-defensins. α-Defensins selectively kill noncommensal bacteria while preserving commensal microbiota. A decrease in the expression of α-defensins may thus result in the loss of microbial diversity. | 63 |
| Allo-HCT | The gastrointestinal mucosa is damaged, and colonizing bacteria are impacted, leading to an impaired intestinal microbiota with reduced diversity. The diversity of the intestinal microbiota at engraftment is an independent predictor of mortality in allo-HCT recipients. Sequencing of free DNA in organ transplant recipients’ plasma revealed an expansion of Anelloviridae upon immunosuppression. There was a lower anellovirus burden in patients who suffer from graft rejection. A similar finding would be expected in the HCT population. | 64, 65 |
Allo-HCT, allogeneic HCT; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HCT, hematopoietic cell transplantation; HCV, hepatitis C virus; HTLV, human T-cell lymphotropic virus; ITP, idiopathic thrombocytopenic purpura; MALT, mucosa-associated lymphatic tissue; PTLD, posttransplantation lymphoproliferative disorder.
Microbiota in hematological diseases
Anemias
Many anemias, such as aplastic anemia and anemia of chronic inflammation, are associated with infections and inflammatory processes, suggesting there may be an important relationship between red blood cells and the microbiota. Aplastic anemia, characterized by pancytopenia and hypoplastic bone marrow, can follow hepatitis A, B, C, E, and G infection and is associated with parvovirus B19, CMV, and EBV.50 In anemia of chronic inflammation, inflammatory cytokines induce hepcidin expression and alter iron homeostasis. Furthermore, infections with various bacterial species have been shown to induce hepcidin.66 Although not yet reported, a direct link between the gut microbiota and hepcidin in anemia of chronic inflammation would be predicted to exist and be of potential clinical significance.
Iron homeostasis, critical in red blood cell metabolism, is also important in regulating bacterial infections. Disorders of iron excess, such as hemochromatosis and chronic hemolysis, render patients susceptible to certain bacterial infections. A randomized controlled study in African children assessed the effects of increased amounts of iron in the colon and discovered significant decreases in lactobacilli, a type of beneficial bacterium, and increased levels of enterobacteria, which includes Escherichia coli and Salmonella species.49 Although existing studies support the role of iron in mediating gut microbiota homeostasis, additional research is necessary to more fully elucidate this relationship.
Lymphomas
Many lymphomas are associated with the presence of specific microorganisms. Relatively strong evidence exists supporting the role of several pathogenic organisms in lymphomagenesis; these organisms include EBV, HCV, Helicobacter pylori, and HIV. Lymphoma of gastric MALT type has been linked to infection with H pylori. Regression of MALT lymphoma has been demonstrated in up to 70% of patients treated with antibiotics that eradicate H pylori infection, suggesting a role for this common gastric microbiota resident in disease persistence.67 In marginal zone lymphomas, HCV is hypothesized to provide the initial antigenic stimulus for B-cell clonal expansion as part of the multistep progression toward lymphomagenesis.56 Other infection-lymphoma relationships have been described between the human T-cell lymphotropic virus 1 and adult T-cell leukemia/lymphoma, between EBV and endemic Burkitt lymphoma and posttransplantation lymphoproliferative disorder, and between human herpesvirus 8 and multicentric Castleman disease.57-60
Platelet disorders
As a rapidly turning-over component of the blood, platelet concentrations are described to be uniquely sensitive to the presence of microbial organisms. Infections have been associated with ITP. For example, there is strong evidence for an association between infection with H pylori and ITP, where platelets can be activated by H pylori antibodies, by FcγIIA, or through an interaction between H pylori–bound von Willebrand factor and platelet glycoprotein IB.68 Other infections that are associated with thrombocytopenia include CMV and varicella zoster virus. CMV can infect megakaryocytes and supporting stroma to produce an acute ITP-like syndrome that occurs in immunocompromised individuals.68 CMV can also cause severe congenital thrombocytopenia and delayed platelet recovery after bone marrow transplantation. HCV-infected patients have a higher incidence of ITP than expected, but the pathophysiology is complex and poorly understood.61
In addition to infection-induced thrombocytopenia, reactive thrombocytosis is a commonly associated condition in the setting of systemic infections. Any inflammatory process, such as bacterial infection or sepsis, that elevates serum interleukin levels (especially IL-6) may increase the circulating platelet count.62 It is important to distinguish this presumably benign reactive condition from malignant thrombocythemic conditions.
Impact of the microbiota on treatment outcome
The composition of the gastrointestinal microbiota, as has been discussed, impacts normal hematopoiesis and can be involved in disease pathogenesis. It is then, perhaps, no surprise that there is evidence supporting the role of the microbiota in mediating treatment response and outcomes in response to chemotherapy, immunotherapy, and stem cell transplantation. Indeed, 2 recent reports demonstrated that the microbiota is a modifier of response to platinum-based chemotherapy, cyclophosphamide, and cytosine guanine dinucleotide oligonucleotide immunotherapy.67,69 Disruption of the microbiota was associated with impaired tumor regression, suggesting that an intact microbiota is required for optimal response to these therapies.
Gastrointestinal dysbiosis in HCT
Immunocompromised patients with inherited, acquired, or iatrogenic disorders are exquisitely sensitive to developing dysbiosis. The type of dysbiosis that can occur varies depending on body site and underlying disorder but, interestingly, is strongly associated with clinical outcomes in certain patient populations. Normally, the human gastrointestinal tract contains a diverse population of microbes, with several types of obligate anaerobic bacteria. During allo-HCT, the gastrointestinal mucosa is damaged and colonizing bacteria are impacted, leading to marked decreases in overall bacterial diversity. In fact, decreased diversity of the intestinal microbiota at engraftment was shown to be an independent predictor of mortality (after multivariate adjustment for other clinical predictors) in allo-HCT recipients.64 The association between low microbiota diversity and mortality was highly significant in a single-institution study; additional investigations in multiple centers will help to clarify the generalizability of this provocative finding. Efforts to understand the potential mechanistic link between loss of microbiota diversity and poor outcomes will also be informative. Although some allo-HCT patients were able to maintain intestinal bacterial diversity throughout the course of transplantation, others had a gut microbiota in which a single bacterial taxon predominated and replaced the previously complex microbial composition.64 One hypothesis linking dysbiosis and mortality is that these dramatic alterations of the intestinal microbiota, in conjunction with neutropenia and mucosal barrier injury, can lead to complications such as septicemia.70 Alternatively, given the strong link between microbiota diversity and the generation of immunologic diversity in the setting of development, another hypothesis is that low microbiota diversity may impact the robustness and speed of immune reconstitution. Another example of the extreme loss of diversity in a subset of post-HCT patients was described in another single-institution study of a cohort of patients with an antibiotic-responsive colitis syndrome. There, next-generation sequencing of colon biopsy samples revealed that the majority of bacterial DNA sequences recovered from the sample were from a novel bacterium, Bradyrhizobium enterica.27 Again, this was a single-institution study; given the significant differences in antibiotic usage (this center used an unusual gut decontamination protocol of neomycin and polymixin), it is difficult to ascertain the generalizability of this organism-disease association to other transplantation centers. However, this finding points out the impressive oligo- or monoclonality of the tissue-invasive or adherent gut microbiota in a subset of post-HCT patients.
GVHD
Another common HCT complication involving the gastrointestinal tract is GVHD. There are many mechanistic models that suggest a role for components of the microbiota in the development of GVHD.71 Low gut microbiota diversity post–allo-HCT is associated with the use of systemic antibiotics, and, in a recent study, this association was more pronounced in gastrointestinal GVHD.72
Several immunologic cell types play a critical role in modulating and responding to the gut microbiota. Antigens from colonic commensal microbiota are important in educating Tregs.73 Tregs and IL-10–producing type 1 regulatory T–like cells compose the major regulatory populations in the intestine.35 Paneth cells, which are located next to intestinal stem cells within the crypts of the intestinal lumen, secrete antimicrobial peptides and α-defensins. α-Defensins are active against many Gram-negative and Gram-positive bacteria, fungi, and enveloped viruses but are typically selectively active against noncommensals. These molecules are hypothesized to function largely by forming pores in bacterial cell walls or by disrupting the cell membrane.74 In GVHD, for example, Paneth cells are lost, resulting in decreased expression of α-defensins, which can lead to an expansion of noncommensal bacteria and decreased microbial diversity.63
In addition to Tregs and Th1 and Th17 cells, the innate immune system also plays a critical role in recognizing pathogens and targeting them for destruction through specialized molecular structures. Pattern recognition receptors are expressed by cells of the innate immune system and are responsible for recognizing pathogen-associated molecular patterns, the evolutionarily conserved components of pathogens. Upon pathogen-associated molecular pattern engagement, pattern recognition receptors trigger intracellular signaling cascades, ultimately culminating in the expression of a variety of proinflammatory molecules, which together orchestrate the early host response to infection. Pathogen-associated molecular pattern engagement and downstream signaling is a prerequisite for the subsequent activation and shaping of adaptive immunity.75 It is hypothesized that these innate immune cells play a critical role in shaping the microbiota through selective targeting of certain microbial organisms. As molecular tools for the dissection of immune cell subsets and microorganisms evolve, it is anticipated that additional mechanisms whereby host-microbe homeostasis is maintained will be identified.
Tools and approaches to modify the microbiota
As more is learned about the microbiota, tools and approaches to modify the microbiota are also being developed in the hope of improving health outcomes. Interventions range from biological therapies such as fecal microbiota transfer (FMT), to modification of the diet, to personalized and targeted antibiotic therapies designed to tailor the exact composition of the microbiota. FMT is the introduction of a fecal suspension derived from a healthy donor into the gastrointestinal tract of a diseased individual. FMT can be performed via the introduction of stool or a purified bacterial stool product via nasogastric tube, colonoscopy, or capsulized therapy. This technique has been used to successfully treat recurrent Clostridium difficile–associated disease.76 FMT results in a change in bacterial composition that is accompanied by resolution of patient symptoms.76 In a recent investigation of capsulized therapy, whereby pills derived from an “optimized human stool source” are administered in 2 successive days, >70% efficacy was achieved with 1 round of therapy; this rate rose to >90% with a second round of therapy. Although FMT has gained traction in specific clinical centers and has been demonstrated to be safe and effective for immunocompetent patients, the application of this therapy has not yet been widely applied to patients with hematologic diseases. Recently, 2 cases of successful FMT have been reported in post–allo-HCT patients; given the very high incidence of C difficile–associated disease in this patient population, FMT provides a potentially attractive treatment approach.77,78 Concerns about infectious organisms transmitted through FMT will need to be addressed prior to broad application of this therapy.
Although pharmacologic and biological approaches to altering the microbiota predominate, another way to influence these microbial communities is through the diet. It has been known for some time that microbial community structures can change drastically in response to long-term differences in dietary intake. Recent studies have shown that short-term consumption of diets composed entirely of animal or plant products can also alter microbial community structure.79 In these studies, alteration of the diet overwhelmed the interindividual differences in microbial gene expression.
It is now evident that humans coexist in an intricate and carefully orchestrated community with the microbiota, in the gut, skin, and beyond. Decades of research have demonstrated that the microbiota composition is closely associated with alterations in human health, and that in some cases, the composition of the microbiota can directly impact health outcomes that range from obesity to immunologic diseases such as allergy and asthma. The development of increasingly refined tools to define the taxonomy, function, and synthetic capacity of the microbiota has potentiated the identification of strong associations between the microbiota and health outcomes. The microbiota can impact human organ systems directly, as well as remotely, even as far as the bone marrow compartment. Although our understanding of the role of the microbiota in normal hematopoiesis and hematologic diseases is at its relative infancy, we anticipate that the tools now available to define the microbiota will lead to the appreciation of the microbiota as a biomarker of disease, with both diagnostic and prognostic utility. In the future, antibiotics may be used to remove or suppress undesirable components of the human microbiome, and probiotics or FMT may be used to introduce or help increase microbial components with known beneficial functions for the human host.80 Given the critical role of the hematologic system in monitoring and adapting to the external environment, we anticipate that investigation of the disease-microbiota relationship will generate new diagnostic, prognostic, and therapeutic approaches for hematologic disorders.
Acknowledgments
This work was supported by National Institutes of Health National Cancer Institute grant K08 CA184420, an American Society of Hematology Scholar Award, the Amy Strelzer Manasevit Award from the National Marrow Donor Program, and an unrestricted gift to the Stanford Microbiome Research Fund from the Samarth Foundation.
Authorship
Contribution: V.E.M. and A.S.B. performed a review of the literature, wrote the paper, and developed the figure.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ami S. Bhatt, Stanford University, 269 Campus Dr, Stanford, CA 94305; e-mail: asbhatt@stanford.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal