In this issue of Blood, Zhou et al provide novel insights into Fc gamma type 2 receptor A (FcγRIIA)–mediated platelet activation. By identifying a negative regulator of FcγRIIA signaling, which is influenced by microRNA miR-148a, they present the first evidence of modulation of platelet function by microRNAs in vivo. They show that inhibition of miR-148a prevents thrombosis after immune complex–induced platelet stimulation in vivo.1
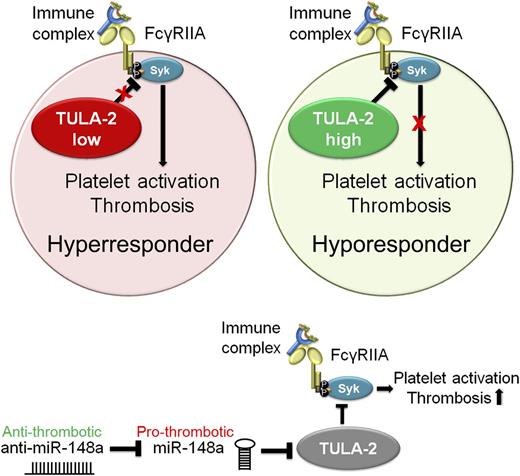
Regulation of FcγRIIA signaling by TULA-2 and miR-148a. Binding of immune complexes to platelet FcγRIIA receptor leads to platelet activation and subsequent thrombotic complications resulting in heparin- and immune-induced thrombocytopenia. Platelet TULA-2 negatively regulates platelet activation downstream of FcγRIIA by diminishing spleen tyrosine kinase (Syk) phosphorylation (P). Intraplatelet TULA-2 levels therefore mediate FcγRIIA-induced platelet activation and thereby determine platelet responsiveness to heparin- or immune-mediated antigen-antibody complexes. In turn, intraplatelet TULA-2 is negatively regulated by microRNA miR-148a. Treatment of mice with anti–miR-148a results in diminished thrombotic complications in vivo.
Regulation of FcγRIIA signaling by TULA-2 and miR-148a. Binding of immune complexes to platelet FcγRIIA receptor leads to platelet activation and subsequent thrombotic complications resulting in heparin- and immune-induced thrombocytopenia. Platelet TULA-2 negatively regulates platelet activation downstream of FcγRIIA by diminishing spleen tyrosine kinase (Syk) phosphorylation (P). Intraplatelet TULA-2 levels therefore mediate FcγRIIA-induced platelet activation and thereby determine platelet responsiveness to heparin- or immune-mediated antigen-antibody complexes. In turn, intraplatelet TULA-2 is negatively regulated by microRNA miR-148a. Treatment of mice with anti–miR-148a results in diminished thrombotic complications in vivo.
Immune-mediated heparin-induced thrombocytopenia (HIT) is an atypical immune complication of heparin treatment that affects 1 in 5000 hospitalized patients. Approximately 50% of these patients develop thromboembolic complications.2 HIT is induced by antibodies that recognize complexes of heparin and platelet factor 4, which trigger platelet activation after binding to platelet FcγRIIA. Interestingly, platelet activation in response to immune complex binding differs significantly between individuals. One possible explanation for these inter-individual variations could be based on the structure and expression levels of FcγRIIA, which determines immune complex binding capacity. However, Zhou et al show that the differences in FcγRIIA activity remain largely unexplained by FcγRIIA expression levels or by a polymorphism that affects immunoglobulin G subclass binding affinity. In particular, individuals who are heterozygous for the principal FcγRIIA polymorphism showed unpredictable platelet responses. This suggests that other factors determine platelet reactivity in response to FcγRIIA-mediated activation.
To address this possibility, Zhou et al1 take a novel approach by investigating potential negative regulators of FcγRIIA signaling that could explain the differences between individuals. They identify a critical role for platelet T-cell ubiquitin ligand-2 (TULA-2) as a negative regulator of FcγRIIA-mediated signaling in platelets. Platelets with low expression levels of TULA-2 strongly respond to immune complexes, whereas high levels of TULA-2 protect from platelet aggregation in response to immune complex binding to FcγRIIA (see figure).
TULA-2, which counteracts the phosphorylation of Syk,3 is a key protein in the downregulation of receptor-mediated signal transduction in platelets in vitro and in vivo. Zhou et al showed that TULA-2 also regulates FcγRIIA-mediated signaling in platelets.
Interestingly, Zhou et al find TULA-2 to be negatively regulated by miR-148a (see figure), one of the 750 microRNAs found within platelets.4 It comes as some surprise that platelets, which are devoid of genomic DNA, are equipped with and regulated by microRNAs. Despite lacking a nucleus, platelets contain messenger RNA (mRNA) splicing machinery and are able to translate mRNA into proteins.5 However, the in vivo contribution of de novo synthesized platelet proteins is incompletely understood. It is also currently unclear whether microRNAs have any function in platelets or if they act solely on the megakaryocyte level.
Several in vitro studies provided solid evidence for a role of microRNAs in megakaryocyte development and platelet production. These modulations could subsequently affect platelet activation and reactivity. Megakaryocytes equip platelets with RNA and microRNA, thereby allowing platelets to mirror the condition of their progenitors. Platelets further take up various RNAs from cells they encounter within the circulation. Platelets also transfer mRNA and microRNAs from one cell to another via release of microvesicles, which allows platelets to communicate with a variety of different cell types, thereby modulating target cell effector functions in various diseases.6 Emerging lines of evidence indicate that platelet microRNAs are biologically and clinically relevant regulators of protein translocation and may serve as biomarkers for thrombotic disease and platelet reactivity, with possible therapeutic applications in the future.4
Zhang et al show for the first time that anti-microRNA therapy can inhibit platelet function in vivo. In an animal model, the authors demonstrate that anti–miR-148a enhances TULA-2 expression in bone marrow cells. This results in a strong reduction of microvessel occlusion and thrombotic events after use of an immune complex–induced in vivo platelet stimulation model. This model mimics the pathological situation of HIT in high responders and demonstrates the importance of TULA-2 function in FcγRIIA-mediated platelet signaling.
Whether the in vivo effects observed by anti–miR-148a are solely the result of FcγRIIA signaling in platelets or whether other cells are also involved remains to be determined. Recently, it was also shown that FcγRIIA signaling in monocytes contributes to thrombotic complications. Monocyte FcγRIIA signaling induces tissue factor production and subsequent thrombin generation in an Syk-dependent fashion.7 Therefore, anti–miR-148a treatment might also have beneficial antithrombotic effects on cell types other than platelets.
The study by Zhou et al brings us one step closer to understanding the underlying mechanism of inter-individual differences in response to FcγRIIA-mediated signaling in platelets. TULA-2 and miR-148a could potentially serve as predictive markers to identify patients at risk of HIT. However, the utility of anti–miR-148a as a therapeutic target is doubtful, because miR-148a has many crucial functions in other cell types. It was previously reported that miR-148a is a tumor-suppressive microRNA that potently induces apoptosis of tumor cells.8 Therefore miR-148a itself has been suggested as a therapeutic target, which, given the effects of this microRNA on platelet activation and subsequent thrombotic complications demonstrated by Zhou et al, should be reconsidered.
Conflict-of-interest disclosure: The author declares no competing financial interests.