Abstract
Introduction Primary HLH (pHLH) is a rare immune regulatory disorder invariably lethal if untreated. It is driven by pathologic immune activation, leading to the development of fever, splenomegaly, cytopenias and coagulopathy, which ultimately may cause multi-organ failure (MOF) and death. Based on data from murine models of primary and secondary HLH (sHLH), and observational studies in patients with HLH, the high production of IFNγ is thought to be a critical factor driving development of the disease. Immune-chemotherapy, primarily etoposide-based regimens, is at present the only pharmacological approach able to control HLH and bring patients to curative allogeneic hematopoietic stem cell transplant (allo-HSCT). In spite of recent attempts to further intensify treatment regimens, mortality and morbidity remain high, in part due to drug-related toxicities.
NI-0501 is a fully human, high affinity, anti-IFNγ mAb that binds to and neutralizes human IFNγ, offering a novel and targeted approach for the control of HLH.
Methods An open-label Phase 2 study has been conducted in United States and Europe to evaluate safety and efficacy of NI-0501 in children with either confirmed or suspected pHLH. NI-0501 was administered at initial dose of 1 mg/kg every 3 days, with possible dose increase guided by PK data and/or clinical response in each patient, on initial background dexamethasone dose of 5-10 mg/m2. Treatment duration ranged from 4 to 8 weeks. Ability to move to allo-HSCT, relevant HLH disease parameters, and 8-week survival were assessed.
Study Population A total of 13 patients were enrolled: 8F/5M, median age 1.0y (range 2.5mo-13y). Twelve patients received NI-0501 as a second line treatment after having received conventional therapy and either reactivating, obtaining unsatisfactory response, or being intolerant to therapy. One patient was treated with NI-0501 in first line. Nine patients carried a known HLH genetic defect (3 FHL2, 2 FHL3, 2 GS-2, 1 XLP1, 1 XLP2). The majority of patients were at the severe end of HLH spectrum, in compromised general condition (2 patients in ICU) and carrying significant toxicities from previous HLH treatments. At baseline ferritin was elevated in 12/13 patients and sCD25 in 8, cytopenias were present in 10 patients, splenomegaly in 8, hypofibrinogenemia and hypertriglyceridemia in 9. Liver and CNS involvement were present in 7 and 3 patients, respectively.
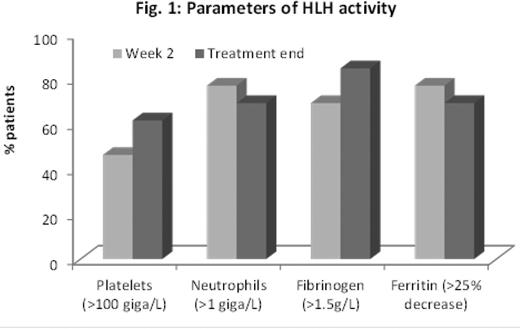
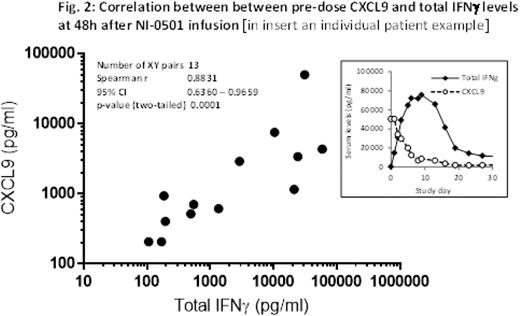
Results Overall, NI-0501 treatment significantly improved parameters of HLH disease activity (Fig. 1), and 9 of 13 patients achieved a satisfactory response. Seven patients have proceeded to allo-HSCT. Allo-HSCT is planned for two patients with good HLH control upon identification of an appropriate donor. In one patient (who achieved disease control with first line NI-0501) allo-HSCT is not yet planned given the absence of a causative HLH gene mutation. Eleven of 13 patients were alive at 8 weeks. Two patients died of HLH/MOF at day 53 and 36, respectively. CNS signs and symptoms resolved in the 2 evaluable patients. Greater than 50% reduction of dexamethasone dose was possible in 50% of patients during the first 4 weeks of NI-0501 treatment. IFNγ neutralization was demonstrated by a sharp decrease in CXCL9, a chemokine exquisitely IFNγ-induced. CXCL9 levels tightly correlated with IFNγ production, estimated by measuring IFNγ bound to NI-0501 (Fig. 2), suggesting CXCL9 as a potential new biomarker for HLH. NI-0501 was well tolerated and no safety concern was identified. None of the infections known to be favored by IFNγ neutralization were reported, and no infections occurred in patients who had not previously received chemotherapy. Seven patients reported at least one SAE, all assessed by the DMC as not related to NI-0501 administration. No unexpected events attributable to “off target” effects of NI-0501 (myelotoxicity, hemodynamic alterations) were observed.
Conclusions Neutralization of IFNγ by NI-0501 offers an innovative targeted and potentially less toxic approach to HLH management. The results of this study show that NI-0501 is a safe and effective therapeutic option in patients with pHLH who have demonstrated unsatisfactory response, or are intolerant, to conventional therapy. Furthermore, therapy with NI-0501 was not associated with any of the typical short- or long-term toxicities reported for etoposide-based regimens. Assessment of NI-0501 as first line treatment for pHLH is ongoing.
Jordan:Novimmune: Consultancy. Allen:Roche: Consultancy , Other: unpaid ; NovImmune: Consultancy , Other: unpaid. De Benedetti:Novimmune: Consultancy. Grom:Novartis: Consultancy , Research Funding ; Novimmune: Consultancy. Ballabio:Novimmune SA: Employment. Ferlin:Novimmune SA: Employment. De Min:Novimmune SA: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal