Abstract
Background: Sickle cell disease is an anemia disorder of the red blood cell in which hemoglobin S (HbS) undergoes polymerization and sickling under low-oxygenation conditions, leading to vaso-occlusive events. Major frequent neurological complications of SCD include ischemia and stroke leading to cognitive deficits and morbidity. Clinical observations have done little to identify the causes of stroke in SCD. Animal models of SCD, and in particular transgenic models have been useful in understanding the microvascular phenomena leading to vasculopathy. Polymerization and consequent sickling of red blood cells (RBC) under oxygen stress conditions likely result in sludging of blood and consequent vasoocclusion, at least in peripheral post-capillary tissues. Most animal studies suggest that the primary site for interaction of RBC's carrying HbSreside in the venous vasculature, yet large arterial stroke remains a significant occurrence in clinical SCD, where suggested risk factors include elevated carotid blood velocity and turbulence, cerebral blood flow asymmetry, loss of cerebrovascular reactivity and cerebral hyperemia. In particular, human cerebral hyperemia appears to be a frequent and significant finding, presumably a compensatory response to the SCD anemia.
We sought to determine how closely Tg animal models of SCD mimic the cerebral findings associated with human SCD. Several models have been developed, including the NY1DD model which does not exhibit anemia, but in which sickling only occurs following a period of hypoxia, the S+S Antilles (SSAnt) model which suffers mild anemia and sickling under normal conditions, and the Berkley (BERK) which exhibits severe anemia, sickling and early mortality. We used MRI to evaluate these SCD models and compared the findings to those of WT animals.
Methods: NY1DD (n=11, 2.6± 0.8 mo, HCT=0.47± 0.04), SSAnt (n=11, 3.3 ± 1.2 mo, HCT=0.43± 0.04), BERK (n=11 at 3 mo, n=5 at 10 mo, HCT=0.33± 0.03) and WT (C57bl, n=5 at 10 mo, n=4) were studied in accord with PHS and AALAC guidelines. Anesthetized (isoflurane) animals were studied with a 9.4 T MRI (Agilent, Inc., CA). MRI included assessment of perfusion (FAIR-ASL), fMRI (BOLD response to a period of hyperoxia), Diffusion Tensor Imaging (DTI), and structural imaging.
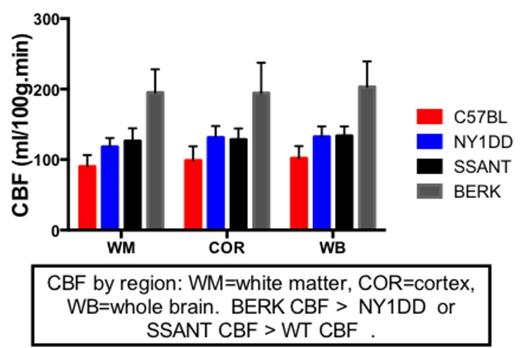
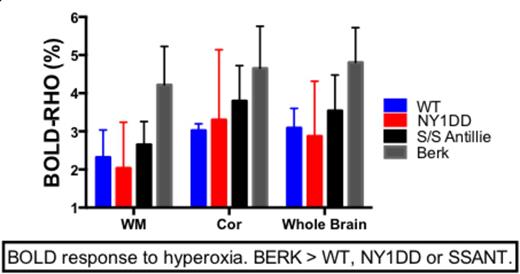
Results: No differences were observed in CBF, BRHO or MD between young and old animals. Cerebral blood flow increased with severity of anemia-with BERK CBF higher than WT, NY1DD or SSAnt CBF (P < 0.0001). NY1DD and SSAnt CBF were not different, but both were higher than WT CBF (p < 0.001). Bold Response to hyperoxia indicated that BERK animals suffered the greatest oxygen debt, while NY1DD and SSAnt were not significantly worse than WT under normoxia conditions. Mean tissue diffusivity (obtained from DTI), a marker for edema and inflammation, was elevated only in BERK mice compared to WT mice (p < 0.05), while fractional anisotropy was not different between groups.
Conclusion: As the degree of anemia worsens, CBF increases presumably to compensate for lower hemoglobin and reduced oxygen carrying capacity. In BERK mice, this increase in CBF is inadequate to compensate for reduced oxygen delivery, and this is indicated by an increase in the BOLD response to hyperoxia (BRHO measure). SSAnt and NY1DD animals both exhibited elevated CBF, but their BRHO was not significantly different from WT animals. This suggests that their increase in CBF was adequate to compensate for sickle induced vasoocclusion and/or mild anemia under normal oxygenation conditions. Our findings suggest that the BERK mouse may be the best model for evaluating therapeutics directed at treating the chronic anemia associated with human SCD, and for evaluating therapeutics aimed at remedying hypoxia induced oxygen radical damage. However, NY1DD or SSAnt may be better models for studying experimentally induced sickle crisis (extended durations of hypoxia), as BERK mice are to fragile to survive such perturbations.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal