Abstract
Introduction: Ibrutinib has shown remarkable safety and efficacy in CLL. Due to CYP3A4 (3A4) metabolism, the concurrent use of 3A4 inhibitors/inducers with ibrutinib should be avoided. Ibrutinib is also associated with bleeding complications, in part due to effects on collagen-mediated platelet aggregation, thus caution is advised with the simultaneous use of anticoagulant (AC) and antiplatelet agents. It is unknown what proportion of CLL patients starting ibrutinib therapy in routine practice are on these medications. We conducted a retrospective study to evaluate these aspects in a large cohort of CLL pts treated with ibrutinib outside the context of clinical trials.
Methods: Following IRB approval, consecutive CLL pts who initiated ibrutinib off-protocol at Mayo Clinic, Rochester, MN from November 2013 through July 2015 were evaluated. A medication review for drug-drug interactions was performed by a pharmacist on all pts. Baseline characteristics, concomitant medications, and toxicity were recorded. Time to toxicity and ibrutinib discontinuation were analyzed by Kaplan Meier and cumulative incidence methods accounting for competing risk of death, respectively.
Results: Ninety-six CLL pts started ibrutinib. Median age was 66 years and 64 (67%) were male. Ibrutinib indications included: relapsed/refractory CLL (n=84), treatment-naïve CLL with del17p (n=8), and CLL with Richter's transformation (n=4). Approximately 80% had unmutated IGHV and 45% had del17p or del11q on FISH. Upon initial pharmacy consult prior to ibrutinib, the median number of concomitant medications was 9 (range, 1-31). Sixty (63%) pts were taking concurrent medications increasing their risk of ibrutinib toxicity and 4 (4%) pts were on drugs potentially reducing ibrutinib efficacy.
At ibrutinib initiation, 16 (17%) pts were on concomitant 3A4 inhibitors. This included 9 (9%) on moderate 3A4 inhibitors (fluconazole, diltiazem, imatinib, cyclosporine) and 7 (7%) on strong 3A4 inhibitors (voriconazole, posaconazole, clarithromycin, itraconazole). In 4 pts, the 3A4 inhibitor was switched to another agent or discontinued to allow standard 420 mg daily dosing. Ibrutinib was initiated at 140 mg daily in 7 pts on moderate 3A4 inhibitors and at 140 mg every 48 hours in 5 pts on strong 3A4 inhibitors.
Four (4%) pts were on strong 3A4 inducers (carbamazepine, rifampin, rifabutin) at the initiation of ibrutinib. The 3A4 inducer was discontinued prior to ibrutinib start in 3 pts, while the initial ibrutinib dose was decreased to 140 mg every 48 hours in the remaining patient (also on 2 strong 3A4 inhibitors).
During the course of ibrutinib, an additional 8 (8%) pts were started on 3A4 inhibitors/inducers which necessitated dose modifications.
Upon commencing ibrutinib, 9 (9%) pts were on concomitant AC (6 warfarin, 3 enoxaparin). Due to significant bleeding risk, warfarin was switched to enoxaparin in 2 pts, to aspirin (ASA) in 1 and warfarin was discontinued in 1. In 2 pts, an alternative AC could not be used so ibrutinib was begun at 140 mg daily and titrated upward.
Twenty-nine (30%) pts were on ASA (3 [3%] also on clopidogrel) at ibrutinib initiation. Nineteen (20%) pts were on selective serotonin release inhibitors (SSRIs), and 9 (9%) were on NSAIDs. Thirteen (13%) pts were on fish oil and 24 (25%) were on herbal medications; all of which were discontinued prior to starting ibrutinib.
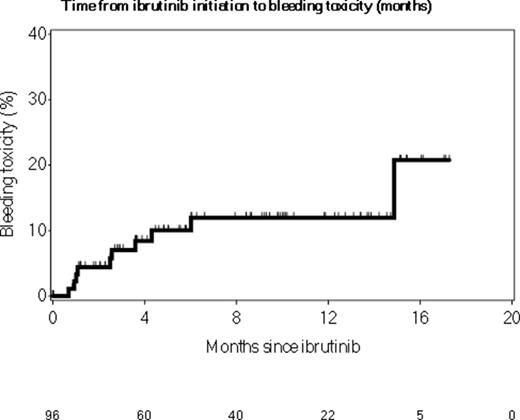
Ten (10%) pts had clinically significant bleeding on ibrutinib including 4 (4%) requiring hospitalization (1 subdural hematoma). Of these 4 pts, 1 was on enoxaparin, 2 were on a SSRI and 1 a NSAID. Six pts had minor bleeding necessitating a dose reduction of ibrutinib to 140-280 mg daily. After 16 months follow-up, the risk of bleeding was 21% (95% CI: 1-37%, Figure).
After a median follow-up of 7.6 months, 73 (76%) pts remain on ibrutinib. There was no difference in rates of discontinuation of ibrutinib between patients who were on 3A4 inhibitors/inducers versus those who were not.
Conclusion: In the "real-world" setting, 2 out of 3 CLL patients commencing ibrutinib therapy is on a concomitant medication with a potentially clinically significant drug-drug interaction with ibrutinib. These findings have implications for the practicing hematologist who must maintain a high degree of vigilance when prescribing ibrutinib to CLL pts. We highly recommend a formal medication review by a clinical pharmacist in all patients initiating ibrutinib.
Ding:Merck: Research Funding. Kay:Hospira: Research Funding; Tolero Pharma: Research Funding; Genentech: Research Funding; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding. Shanafelt:Glaxo-Smith_Kline: Research Funding; Pharmactckucs: Research Funding; Celgene: Research Funding; Genentech: Research Funding; Cephalon: Research Funding; Janssen: Research Funding; Hospira: Research Funding; Polyphenon E Int'l: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal