Abstract
It is now generally believed that proliferation of the neoplastic clone in chronic lymphocytic leukemia (CLL) takes place in lymphoid tissues where interactions involving the B-cell receptor (BCR) and other microenvironmental elements take place. Previous studies using in-vivo labelling with deuterated water have shown that recently proliferated emigrants from lymphoid tissues express low levels of the chemokine receptor CXCR4 and high levels of CD5 (CXCR4loCD5hi). It has been proposed that, following entry into the peripheral blood (PB), these cells become quiescent, re-express CXCR4 and downregulate CD5 allowing re-entry into tissues and further rounds of proliferation. In the present study we used in-vivo labelling with deuterated glucose (6,6-2H2-glucose, D2G) to investigate the proliferation and release of CLL cells into PB. In contrast to deuterated water, this technique allows pulse labelling of a distinct cohort of cells, which can then be tracked over time in-vivo.
Labelling studies were performed in 10 patients with previously untreated, non-progressive CLL. Patients underwent 10 hours of labelling with oral 2DG, after which peripheral blood and lymph node compartments were serially sampled. DNA Deuterium enrichment was measured in the entire CLL population and in flow-sorted subsets defined by CXCR4/CD5 and surface IgM (sIgM) expression.
Maximum release of labelled cells into PB occurred after a median of 14 days (4-56 days). The disappearance rate was very slow, with labelled cells detectable after 56 days in half of the subjects. In one case we were able to track labelled cells in both lymph node (LN) and PB and demonstrated an increase in the fraction of labelled cells in the LN between days 7 and 28, consistent with re-entry into this compartment.
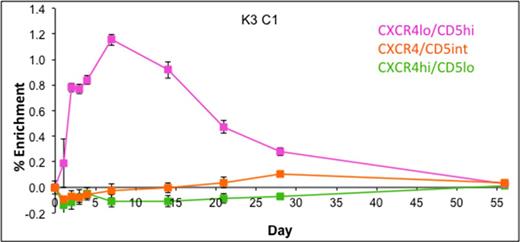
Subpopulations of PB CLL cells, defined by CXCR4 and CD5 expression were studied over time to investigate the dynamic nature of these molecules in circulating cells. As previously reported, maximum rapid incorporation of deuterium was observed in the CXCR4loCD5hi fraction, equivalent to 1.15 ± 0.04 %/d fractional synthesis at 7 days. Conversely, CXCR4hiCD5lo cells remained largely unlabelled throughout the 8-week study, reaching a maximum of only 0.01 ± 0.003 %/d, suggesting that they represent a non-proliferating population, not derived from the CXCR4loCD5hi subset. In contrast, CXCR4/CD5 intermediate cells exhibited delayed and intermediate labelling, peaking at 0.1 ± 0.02 %/d at 28 days. This sequential labelling pattern suggests that they derive from the CXCR4loCD5hi subset.
Since both CXCR4 and CD5 expression are modulated by BCR signalling, we went on to sort cells according to sIgM expression and found maximum labelling in the sIgM high subset with little or no deuterium incorporation in the sIgM low fraction at any time. Again, the sIgM intermediate subset showed delayed labelling, suggesting that they are derived from sIgM-high cells.
These observations provide further evidence for clonal heterogeneity in CLL and suggest the existence of distinct but interdependent subpopulations. Recently-divided cells are CXCR4loCD5hi, but appear to transition to an intermediate phenotype by down-regulation of CD5 and sIgM and upregulation of CXCR4 over the ensuing weeks, but do not appear to transition to CXCR4hiCD5lo cells over the time-course of the experiment. Our findings have clinical relevance, since these functionally distinct subsets might also differ in their responsiveness to therapeutic agents, such as drugs that block BCR signalling.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal