Abstract
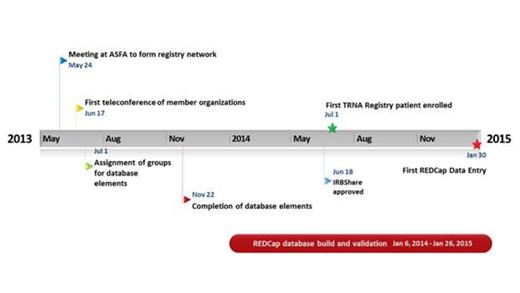
The thrombotic microangiopathies (TMAs) are rare, life-threatening thrombotic disorders of diverse etiologies. Systematic studies of TMA have been difficult to perform due to their rare occurrence, disease heterogeneity and, lack of an organized research network in the United States. Whereas a multi-institutional approach has been used in Europe and Canada, TMA research in the US has been largely single-center based. To overcome the limitations of single institutional registries and to expand the number of TMA patients available for study, four academic centers convened during the 2013 ASFA annual meeting to establish the TMA Registry of North America (TRNA) with the following goals: (1) design and develop a registry to define "best practices" for the diagnosis, treatment, and management of TMA; (2) develop a platform for conducting observational and interventional clinical trials; (3) and, establish a national bio-repository of samples from patients with TMA to facilitate future studies. This abstract reports the first multi-institutional network in the United States designed to study TMA. Members met through bimonthly tele-conferences to develop a clinical registry using REDCap (Research Electronic Data Capture), a HIPAA-compliant, internet-based software program for data entry. To facilitate a cohesive and streamlined review of IRB applications, the TRNA utilized IRBshare, a portal for rapid approval of multi-site investigations. IRB consent included participation in a bio-repository arm for collecting blood and apheresate. Following approval through the Duke IRB in June 2014, study documents were uploaded to the IRBshare website. Since August 2015, 16 study participants with TMA have been consented, 13 of which have had their clinical information entered into the database. Preliminary data shows our population is 73% African-American and female. Average laboratory values at presentation included hemoglobin of 9.7 g/dL, platelets 53 x10^9/L, and LDH 1,150 u/L. ADAMTS13 testing was performed in 77% (10/13), of which 60% (6/10) measured at <10%. Current efforts include expansion of network sites to expand the registry and to develop clinical protocols for future studies. With its clinical and IRB infrastructure in place, the TRNA, which is the first U.S. research network designed to study TMA, is poised to perform cooperative observational and interventional trials in the near future.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal