Abstract
Autologous stem cell transplantation (SCT), a standard therapy for patients with multiple myeloma (MM), has been offered continuously for over 25 years at our center. During this time, there have been significant advances in therapy and marked improvements in survival. We performed a single-center retrospective study comparing patients transplanted from 1988-2000 (cohort A) and 2001-2012 (cohort B). We evaluated differences at, toxicities of, and outcomes with and after SCT. This comparison highlights the contributions of advances in SCT-related conditioning regimens, supportive care and the impact of new therapeutic agents on patient outcomes.
Data were extracted from two internal clinical and stem cell databases and from medical chart reviews of all patients undergoing SCT for MM from 1988-2012. Disease responses were assessed with IMWG response criteria. The National Death Index was queried for patients lost to follow-up, without date of death, or without cause of death. Progression-free (PFS) and overall survival (OS) were defined as time from SCT to progression and/or death and estimated by Kaplan-Meier (two-sided log rank test). Patients with a second SCT or allogeneic SCT were censored at the date of second SCT. Multivariate analysis was performed using Cox's Proportional Hazard Regression models. We calculated adjusted survival probabilities by employing a stepwise model selection to identify significant covariates. Factors that were significant at 0.15 level were kept in the final model.
Patient characteristics of the cohorts (A=63, B=116) including gender, lab values at diagnosis, renal and skeletal involvement, M-protein isotypes, staging, pre-SCT screening and infectious disease testing, were no different. Cohort A was younger at SCT (54 vs 57 years) and transplanted later post-diagnosis. More in cohort A had been treated with melphalan (MEL) (48% vs 4%) and palliative radiation (XRT) to bone lesions (54% vs 26%) pre-SCT. MM disease status was significantly different at SCT for A and B (Table 1). Three quarters of patients in cohort A were mobilized with cyclophosphamide and G-CSF (Cy-G) but 23% were collected in steady-state or after GM-CSF, while 99% in cohort B were mobilized with Cy-G or G alone. Notable aspects of conditioning regimens and peri-transplant complications are shown in Table 1. Exposure to prior XRT in cohort A correlated with development of secondary MDS/AML.
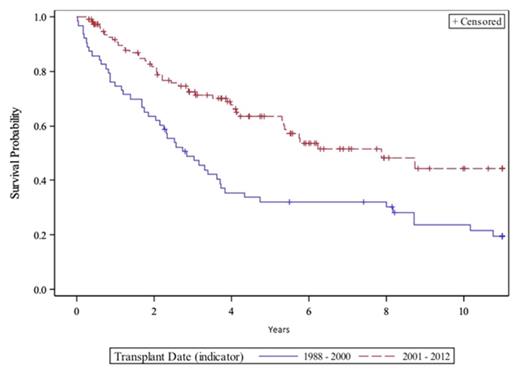
OS post-SCT differed between the two cohorts (2.8 vs 7.9 years, P < 0.01) (Figure 1). No patients in cohort A received a novel agent pre-SCT compared to 68% in cohort B including >30% of cohort B receiving pre-SCT bortezomib. CR/VGPR status at SCT predicted improved OS (P = 0.04) while pre-SCT exposure to oral MEL predicted worse OS (HR 1.97, P = 0.02). Patients in cohort A died shortly after post-SCT relapse, whereas those in cohort B lived longer after relapse (1.0 vs 2.7 years, P < 0.01). Median OS of patients who received a novel agent at any time was not reached compared to 3.4 years for others. Cause of death differed between the two cohorts. More patients died of MM progression in cohort B (80% vs 58%), while more patients died of transplant related causes or secondary MDS in cohort A (19% vs 2%).
In sum, we demonstrate a clear improvement in outcomes of MM patients selected to undergo first SCT over the past 25 years. OS has improved alongside reductions in TRM. Novel agents have contributed to these improvements although advances in SCT and supportive care are also important contributors. Further research into more efficacious treatments, such as monoclonal antibody therapy, is required to continue the improvements in SCT for MM.
| Variable . | Cohort A 1988 - 2000 (N = 63) (%) . | Cohort B 2001 - 2012 (N = 116) (%) . | P -value . |
|---|---|---|---|
| Months from diagnosis to SCT | 12.7 (5.6-95.3) | 8.9 (4.2-50.6) | < 0.01 |
| Patients > 64 years old | 3 (5) | 19 (16) | 0.024 |
| MM status at SCT | |||
| CR | 15 (24) | 31 (27) | < 0.01 |
| VGPR | 2 (3) | 27 (23) | |
| PR | 29 (48) | 40 (34) | |
| SD | 5 (8) | 9 (8) | |
| Refractory | 6 (10) | 5 (4) | |
| Relapsed | 5 (8) | 4 (3) | |
| Conditioning regimen | |||
| MEL 140 | 7 (11) | 15 (13) | < 0.01 |
| MEL 200 | 2 (3) | 99 (85) | |
| MEL 140/TBI | 41 (65) | 2 (2) | |
| MEL 140/TBI/Cy | 12 (19) | 0 (0.00) | |
| MEL 140/Cy | 1 (2) | 0 (0.00) | |
| Days in hospital for SCT | 24 (13 - 64) | 20 (11 - 36) | < 0.01 |
| Failure to engraft for platelets | 9 (14) | 1 (1) | < 0.01 |
| Treatment-related mortality (100d) | 6 (10) | 1 (1) | < 0.01 |
| MM response post-ASCT | |||
| CR | 26 (46) | 59 (53) | < 0.01 |
| VGPR | 6 (11) | 31 (28) | |
| PR | 21 (38) | 18 (16) | |
| SD | 2 (4) | 4 (4) | |
| Relapsed | 1 (2) | 0 (0) | |
| Secondary MDS/AML | 6 (10) | 0 (0) | < 0.01 |
| Variable . | Cohort A 1988 - 2000 (N = 63) (%) . | Cohort B 2001 - 2012 (N = 116) (%) . | P -value . |
|---|---|---|---|
| Months from diagnosis to SCT | 12.7 (5.6-95.3) | 8.9 (4.2-50.6) | < 0.01 |
| Patients > 64 years old | 3 (5) | 19 (16) | 0.024 |
| MM status at SCT | |||
| CR | 15 (24) | 31 (27) | < 0.01 |
| VGPR | 2 (3) | 27 (23) | |
| PR | 29 (48) | 40 (34) | |
| SD | 5 (8) | 9 (8) | |
| Refractory | 6 (10) | 5 (4) | |
| Relapsed | 5 (8) | 4 (3) | |
| Conditioning regimen | |||
| MEL 140 | 7 (11) | 15 (13) | < 0.01 |
| MEL 200 | 2 (3) | 99 (85) | |
| MEL 140/TBI | 41 (65) | 2 (2) | |
| MEL 140/TBI/Cy | 12 (19) | 0 (0.00) | |
| MEL 140/Cy | 1 (2) | 0 (0.00) | |
| Days in hospital for SCT | 24 (13 - 64) | 20 (11 - 36) | < 0.01 |
| Failure to engraft for platelets | 9 (14) | 1 (1) | < 0.01 |
| Treatment-related mortality (100d) | 6 (10) | 1 (1) | < 0.01 |
| MM response post-ASCT | |||
| CR | 26 (46) | 59 (53) | < 0.01 |
| VGPR | 6 (11) | 31 (28) | |
| PR | 21 (38) | 18 (16) | |
| SD | 2 (4) | 4 (4) | |
| Relapsed | 1 (2) | 0 (0) | |
| Secondary MDS/AML | 6 (10) | 0 (0) | < 0.01 |
Relias:Genentech: Speakers Bureau. Miller:Biogen Idec: Consultancy; AbbVie: Speakers Bureau; Millennium: Speakers Bureau; Onynx: Speakers Bureau. Comenzo:Prothena: Research Funding; Karyopharm: Research Funding; Janssen: Research Funding; Takeda Millennium: Membership on an entity's Board of Directors or advisory committees; Takeda Millennium: Research Funding; Prothena: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal