Abstract
Background: Recent data have shown that pre-transplant FDG-PET is prognostic in DLBCL patients undergoing autologous stem cell transplantation (ASCT). We retrospectively analyzed data on patients with DLBCL treated with ASCT to assess the impact of pre-transplant FDG-PET on relapse-free survival (RFS) and overall survival (OS).
Methods: We reviewed medical records of 32 patients with DLBCL who underwent ASCT using high dose busulfan, cyclophosphamide, and etoposide (Bu/Cy/VP) at Cleveland Clinic from June 2008 to December 2012 and had both a relapse FDG-PET as well as a pre-transplant FDG-PET available for review. All images were interpreted by a staff nuclear medicine radiologist blinded to the outcomes. Visual analysis was performed using the Deauville five-point scale and semiquantitative analysis was done by measuring the maximum standardized uptake value (SUVmax). ΔSUVmax was calculated by determining the difference between the SUVmax at the time of relapse and the SUVmax immediately prior to transplant. Patients were grouped into pre-transplant Deauville score 1-3 or 4-5. Baseline characteristics were compared between groups using the Chi-square test or Wilcoxon rank test. Cox proportional hazards analysis was used to identify prognostic factors. Outcomes were calculated from the date of ASCT. Overall survival (OS) and relapse-free survival (RFS) were estimated using the Kaplan-Meier method and compared using the log-rank test
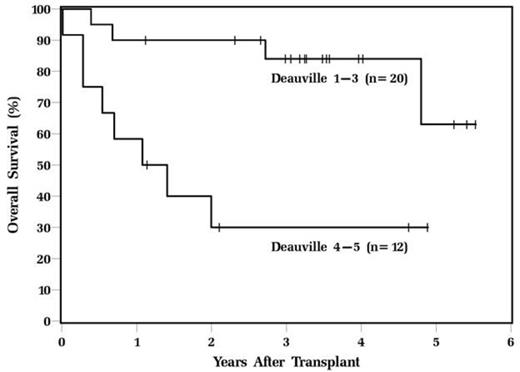
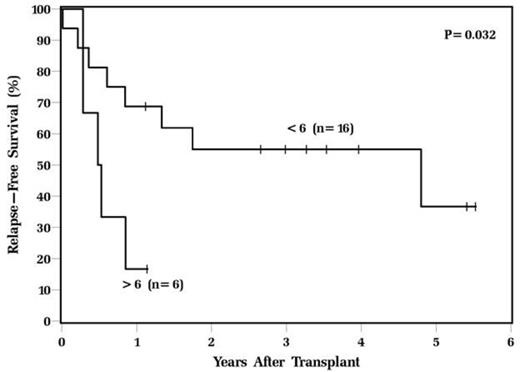
Results: The median age of the patients at transplant was 57 and 69% were male. There was no significant difference in baseline characteristics of patients who had a Deauville score 1-3 compared to patients who had a Deauville score 4-5 including mean age, gender, race, Karnofsky performance status, number of prior chemotherapy regimens, prior radiation therapy, and IPI at diagnosis and transplant. There was a trend towards significance in the median SUVmax at relapse in the Deauville 1-3 group compared to the Deauville 4-5 group (11.1 vs. 18.1, p=0.08) and a significant difference in the median pre-transplant SUVmax (2.3 vs. 8.1, p<0.001). Deauville score 4-5 was the only baseline variable that was prognostic for both RFS (HR=4.2, CI 1.6-11.5, p=0.004) and OS (HR=5.5, CI 1.6-18.9, p=0.006). The 3-year RFS for patients in the Deauville 1-3 group was 64% compared to 12% in the Deauville 4-5 group (p=0.002) and the 3-year OS for patients in the Deauville 1-3 group was 84% compared to 30%, respectively (p=0.002 [Figure 1]). The median ΔSUVmax was 74%. There was no difference in RFS or OS in patients who had a ΔSUVmax above or below this median. However, a high pre-transplant SUVmax(>6 vs. <6) was associated with significantly inferior RFS (p=0.03 [Figure 2]).
Conclusions: Pre-transplant FDG-PET Deauville score is prognostic of RFS and OS in patients with DLBCL undergoing ASCT. High SUVmax(>6) prior to transplant is predictive of poor RFS. Pre-transplant PET is a powerful tool for identifying DLBCL patients at high risk for treatment failure with ASCT and could be used to risk-stratify patients in prospective clinical trials of novel transplant strategies.
OS of DLBCL patients undergoing ASCT based on pre-transplant Deauville score.
RFS of DLBCL patients undergoing ASCT based on pre-transplant SUVmax.
Smith:celegene, spectrum, genentech: Honoraria. Majhail:Gamida Cell Ltd.: Consultancy; Anthem Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal