Abstract
Introduction: Pleural effusions are common clinical finding in patients undergoing hematopoietic stem cell transplantation (HSCT). It can be a manifestation of underlying lung injury or a part of a systemic process. This study will investigate the incidence, risk factors, and clinical outcomes associated with pleural effusion in allogeneic HSCT.
Methods: We conducted a retrospective study of 618 consecutive adult patients who underwent allogeneic HSCT for hematological disorders, between January 2008 and December 2013.
Results: The baseline characteristics of the groups with and without pleural effusions are shown in table 1. The only significant difference was GVHD prophylaxis (p =0.002).
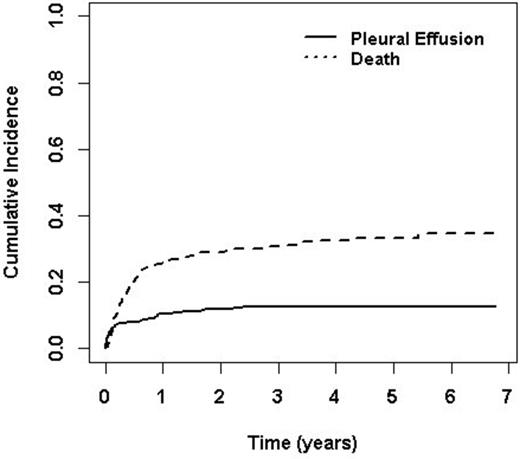
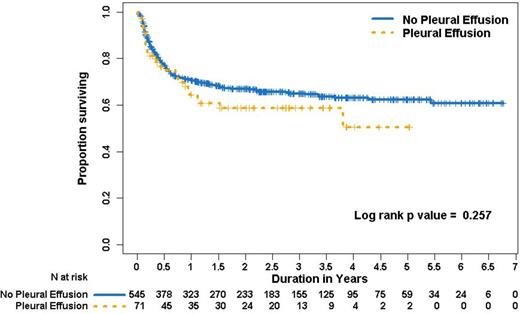
A total 71 patients developed pleural effusions with the cumulative incidence of 12.6 % (95% CI, 10.0% to 15.6%) in five years. The median time of onset of pleural effusion from HSCT was 40 days (range, 1, 869). The onset of pleural effusion had a bimodal distribution with the first peak at 23 days (range 17, 29) and second peak at 362 days (range 277, 450). Based on chest CT criteria, the effusions were judged as large, moderate and small in size in 15%, 51% and 34% of patients respectively. Twenty five (35%) patients with pleural effusions underwent thoracentesis for respiratory difficulty and had a median of 800cc (range, 250 to 13500cc) of pleural fluid removed. Fourteen patients (56%) had exudative and 9 (36%) had transudative pleural effusions. Pleurx catheter was placed in 2 (3%), and chest tube in 3 (4%) patients. Causes of effusion listed in order of frequency are infection (38%), volume overload (23%), chronic GVHD (20%), heart failure (6%), engraftment syndrome (4%), veno-occlusive disease (4%), malignant pleural effusion (3%), hypersensitivity pneumonitis (1%) and iatrogenic (1%). Time of onset is different among various etiologies (p=0.006). Although thoracentesis was performed in 25 patients, the specific etiology by pleural fluid analysis was identified in only 2 patients. Pleural effusion secondary to infections, hypersensitivity pneumonitis, and engraftment syndrome occurred in the first 100 days after transplant, whereas pleural effusion due to chronic GVHD/polyserositis, bronchiolitis obliterans occurred 200 days after transplant.Fifty seven patients (80%) with pleural effusions had evidence of GVHD however; fourteen (20%) patients with pleural effusion had no evidence of acute or chronic GVHD. Twenty three (32%) patients had both pleural effusion and ascites, 27 (38%) had both pleural and pericardial effusion and 29 patients had pleural effusion alone. Multivariate cox regression analysis showed age, African American race, higher comorbidity index, unrelated donor, HLA-match 7/8, intermediate to high risk disease to be associated with worse survival among patients who developed pleural effusion after HSCT. There was no significant difference in overall survival between patients with or without pleural effusion (p=0.257).
Conclusion: Majority of the patients who developed pleural effusions responded well with the treatment of the underlying disease. Although thoracentesis was often done for therapeutic reasons, it was a weak diagnostic tool. We did not identify any adverse impact of development of post HSCT pleural effusions on overall survival.
| Characteristics . | . | Pleural Effusion (N=71) . | No Pleural Effusion (N=547) . | Signif . | |
|---|---|---|---|---|---|
| Age - Median (range) year | 55(25,73) | 58(22,78) | 0.514 | ||
| Sex - no. (%) | 0.522 | ||||
| Male/Female | 43(61)/28(39) | 305(56)/242(44) | |||
| Diagnosis - no. (%) | 0.490 | ||||
| AML | 25(35) | 213 (39) | |||
| ALL | 9(13) | 53 (10) | |||
| CLL | 4(6) | 27 (5) | |||
| CML | 2 (3) | 16 (3) | |||
| NHL | 13 (18) | 94 (17) | |||
| Multiple Myeloma | 3 (4) | 16 (3) | |||
| MDS | 9 (13) | 74 (14) | |||
| Myelofibrosis | 1 (1) | 18 (3) | |||
| Hodgkin's Lymphoma | 1 (1) | 8 (1) | |||
| PLL | 4 (6) | 7 (1) | |||
| Aplastic Anemia | 0 (0) | 17 (3) | |||
| CMML | 0 (0) | 4 (1) | |||
| Comorbidity-Median (range) | 3 (0,8) | 3 (0,9) | 0.055 | ||
| Disease risk status - no. (%) | 0.389 | ||||
| Low | 4 (6) | 48 (9) | |||
| Intermediate | 32 (45) | 284 (52) | |||
| High | 31 (44) | 193 (35) | |||
| Very High | 4 (6) | 22 (4) | |||
| HLA - no. (%) | 0.566 | ||||
| 8/8 | 56 (79) | 413 (76) | |||
| 7/8 | 12 (17) | 117 (21) | |||
| <7/8 | 3 (4) | 17 (3) | |||
| Donor - no. (%) | > 0.99 | ||||
| Matched related | 25 (35) | 197 (36) | |||
| Matched unrelated | 46 (65) | 350 (64) | |||
| Conditioning regimen - no. (%) | 0.761 | ||||
| Full intensity | 45 (63) | 361 (66) | |||
| Reduced intensity | 26 (37) | 186 (34) | |||
| GVHD prophylaxis - no. (%) | 0.002 | ||||
| Mycophenolate-Tacrolimus | 41 (58) | 328 (60) | |||
| Mycophenolate-Tacrolimus-Thymoglobulin | 26 (37) | 131 (24) | |||
| Tacrolimus- Thymoglobulin | 3 (4) | 17 (3) | |||
| Thymoglobulin-Tacrolimus-Sirolimus | 0 (0) | 64 (12) | |||
| Others | 0 (0) | 5 (1) | |||
| Characteristics . | . | Pleural Effusion (N=71) . | No Pleural Effusion (N=547) . | Signif . | |
|---|---|---|---|---|---|
| Age - Median (range) year | 55(25,73) | 58(22,78) | 0.514 | ||
| Sex - no. (%) | 0.522 | ||||
| Male/Female | 43(61)/28(39) | 305(56)/242(44) | |||
| Diagnosis - no. (%) | 0.490 | ||||
| AML | 25(35) | 213 (39) | |||
| ALL | 9(13) | 53 (10) | |||
| CLL | 4(6) | 27 (5) | |||
| CML | 2 (3) | 16 (3) | |||
| NHL | 13 (18) | 94 (17) | |||
| Multiple Myeloma | 3 (4) | 16 (3) | |||
| MDS | 9 (13) | 74 (14) | |||
| Myelofibrosis | 1 (1) | 18 (3) | |||
| Hodgkin's Lymphoma | 1 (1) | 8 (1) | |||
| PLL | 4 (6) | 7 (1) | |||
| Aplastic Anemia | 0 (0) | 17 (3) | |||
| CMML | 0 (0) | 4 (1) | |||
| Comorbidity-Median (range) | 3 (0,8) | 3 (0,9) | 0.055 | ||
| Disease risk status - no. (%) | 0.389 | ||||
| Low | 4 (6) | 48 (9) | |||
| Intermediate | 32 (45) | 284 (52) | |||
| High | 31 (44) | 193 (35) | |||
| Very High | 4 (6) | 22 (4) | |||
| HLA - no. (%) | 0.566 | ||||
| 8/8 | 56 (79) | 413 (76) | |||
| 7/8 | 12 (17) | 117 (21) | |||
| <7/8 | 3 (4) | 17 (3) | |||
| Donor - no. (%) | > 0.99 | ||||
| Matched related | 25 (35) | 197 (36) | |||
| Matched unrelated | 46 (65) | 350 (64) | |||
| Conditioning regimen - no. (%) | 0.761 | ||||
| Full intensity | 45 (63) | 361 (66) | |||
| Reduced intensity | 26 (37) | 186 (34) | |||
| GVHD prophylaxis - no. (%) | 0.002 | ||||
| Mycophenolate-Tacrolimus | 41 (58) | 328 (60) | |||
| Mycophenolate-Tacrolimus-Thymoglobulin | 26 (37) | 131 (24) | |||
| Tacrolimus- Thymoglobulin | 3 (4) | 17 (3) | |||
| Thymoglobulin-Tacrolimus-Sirolimus | 0 (0) | 64 (12) | |||
| Others | 0 (0) | 5 (1) | |||
Deol:Bristol meyer squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal